Convergence and divergence in the neurochemical regulation of prepulse inhibition of startle and N40 suppression in rats - PubMed (original) (raw)
Convergence and divergence in the neurochemical regulation of prepulse inhibition of startle and N40 suppression in rats
Neal R Swerdlow et al. Neuropsychopharmacology. 2006 Mar.
Abstract
Prepulse inhibition of startle ('PPI'), a cross-species measure of sensorimotor gating, is impaired in schizophrenia patients. Suppression of P50 event-related potentials (ERPs) in response to the second of two clicks ('P50 gating') is also impaired in schizophrenia. Suppression of N40 ERPs to the second of two clicks ('N40 gating') is thought by some to be a rat homolog of human P50 gating. Emerging evidence suggests differences in the neurobiology of deficits detected by PPI vs P50 (or N40) gating. We recorded PPI and N40 gating contemporaneously in rats, to assess convergence and divergence in the neurochemical regulation of these measures. Dose-response studies examined the effects of apomorphine (APO), phencyclidine (PCP) or the 5HT2A agonist DOI on PPI, and on motor responses to stimuli (S1 and S2) that elicit N40 gating. Effects of optimal drug doses on PPI and N40 gating were then assessed in other rats with implanted cortical surface electrodes. APO, PCP and DOI caused dose-dependent disruptions of both PPI and gating of motor responses to N40 stimuli. Reduced PPI reflected diminished prepulse effectiveness, demonstrated by increased startle levels on prepulse+pulse trials. In contrast, reduced gating of motor responses to N40 stimuli reflected a reduced motor response to S1. In separate rats, robust PPI, N40 potentials and N40 gating could be detected within one test. PPI and N40 gating were disrupted by APO, PCP, and DOI. Again, drug effects on PPI reflected increased startle on prepulse+pulse trials, while those on N40 gating reflected reduced ERP responses to S1. In conclusion, when PPI and N40 gating were studied concurrently in rats, drug effects on PPI reflected reduced inhibition of startle by the prepulse, while diminished N40 gating reflected S1 response suppression. Despite similarities in drug sensitivity, these results suggest that distinct neurobiological mechanisms underlie drug-induced deficits in PPI and N40 gating.
Figures
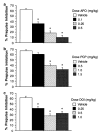
Figure 1
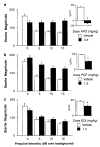
Percent PPI in dose–response studies with APO (a), PCP (b) and DOI (c). (*) p < 0.05 vs vehicle dose.
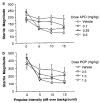
Figure 2
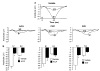
Raw startle magnitude on PULSE trials (0 dB prepulse) and trials in which PULSE was preceded by 5, 10, or 15 dB prepulses, in rats treated with APO (a) or PCP (b). Note that in both (a) and (b): (1) vehicle-treated rats exhibit an orderly, intensity-dependent suppression of startle, and (2) rats treated with active drug doses exhibit a dose-dependent reduction in sensitivity to the startle-inhibiting effects of prepulses. This drug-induced reduction in the ability of prepulses to inhibit startle provides clear evidence for a loss of sensorimotor gating.
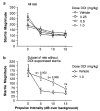
Figure 3
Raw startle magnitude on PULSE trials (0 dB prepulse) and trials in which PULSE was preceded by 5, 10, or 15 dB prepulses, in rats treated with DOI. (a) DOI produces a dose-dependent reduction in startle magnitude on PULSE trials that complicates a clear interpretation of drug effects on sensorimotor gating. (b) Startle magnitude from a subgroup of rats whose startle magnitude on PULSE trials was not significantly reduced by DOI (1.0 mg/kg). Note that for this group, a DOI-induced loss of sensorimotor gating is evident via the reduced ability of prepulses to inhibit startle magnitude (dose × trial interaction: F = 6.05, df 3,15, p < 0.007; posthoc comparisons: p < 0.002 (5 dB) and p < 0.008 (10 dB)). A comparable or slightly diminished separation was observed for the 0.5 mg/kg dose of DOI (p < 0.002 (5 dB) and p < 0.04 (10 dB)), but not for the 0.25 mg/kg dose (NS, all prepulse intensities).
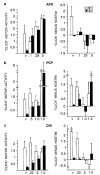
Figure 4
Motor activity on trials with S1 and S2 ‘clicks’. Left side of figure shows ‘click motor activity’: peak response units 100 ms after S1 or S2 ‘clicks’ on same scale as startle magnitude in Figures 2–3. Note that motor activity on ‘click’ trials in vehicle-treated rats was: (1) 0.5–1.0% of the magnitude exhibited in response to PULSE trials; (2) greatly suppressed on S2 vs S1 trials. Also note that S2 amplitude appeared to be enhanced preferentially in a dose-dependent manner by (a) APO, (b) PCP, and (c) DOI. Right side of figure shows the same data, minus response units exhibited on NOSTIM trials (‘click minus nostim’). Note that, by removing NOSTIM background activity, S2 ‘gating’ remains intact in vehicle-treated rats, and the most evident drug effect is a suppression of motor activity in response to S1 clicks.
Figure 5
Raw startle magnitude on PULSE trials (0 dB prepulse) and trials in which PULSE was preceded by 5, 10, or 15 dB prepulses, in rats treated with vehicle or a single dose of APO (a), PCP (b), or DOI (c). Note comparability to Figures 2–3. Insets reveal mean %PPI, collapsed across prepulse intensities (*p < 0.05 vs vehicle). APO study used a within-subject design, while PCP and DOI studies used between-subject designs in order to limit total number of tests for each rat.
Figure 6
N40 ERP responses. (a) Grand average ERP waveforms in response to S1 and S2, across all rats treated with vehicle (top), APO, PCP, or DOI. Note in vehicle treated rats, the peak negativity to S1 at 40 ms poststimulus (stimulus at arrow), and reduced (‘gated’) amplitude to S2. This gating was disrupted by APO, PCP, and DOI, as a result of reduced S1 amplitude. This was evident in grand average waveforms, and in peak N40 amplitudes, shown in b. (b) Peak N40 amplitudes in response to S1 and S2 stimuli, in rats treated with vehicle or APO (left), PCP (middle), or DOI (right). Note significant reduction in S1 amplitude after treatment with APO and DOI (*p < 0.05 vs vehicle), and similar trend for this effect after treatment with PCP.
Similar articles
- Sensory and sensorimotor gating-disruptive effects of apomorphine in Sprague Dawley and Long Evans rats.
Breier MR, Lewis B, Shoemaker JM, Light GA, Swerdlow NR. Breier MR, et al. Behav Brain Res. 2010 Apr 2;208(2):560-5. doi: 10.1016/j.bbr.2009.12.037. Epub 2010 Jan 18. Behav Brain Res. 2010. PMID: 20080128 Free PMC article. - Failure of haloperidol to block the effects of phencyclidine and dizocilpine on prepulse inhibition of startle.
Keith VA, Mansbach RS, Geyer MA. Keith VA, et al. Biol Psychiatry. 1991 Sep 15;30(6):557-66. doi: 10.1016/0006-3223(91)90025-h. Biol Psychiatry. 1991. PMID: 1834231 - Sensory and sensorimotor gating deficits after neonatal ventral hippocampal lesions in rats.
Swerdlow NR, Light GA, Breier MR, Shoemaker JM, Saint Marie RL, Neary AC, Geyer MA, Stevens KE, Powell SB. Swerdlow NR, et al. Dev Neurosci. 2012;34(2-3):240-9. doi: 10.1159/000336841. Epub 2012 May 8. Dev Neurosci. 2012. PMID: 22572564 - Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review.
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Geyer MA, et al. Psychopharmacology (Berl). 2001 Jul;156(2-3):117-54. doi: 10.1007/s002130100811. Psychopharmacology (Berl). 2001. PMID: 11549216 Review. - Prepulse inhibition of the acoustic startle reflex and P50 gating in aging and alzheimer's disease.
Jafari Z, Kolb BE, Mohajerani MH. Jafari Z, et al. Ageing Res Rev. 2020 May;59:101028. doi: 10.1016/j.arr.2020.101028. Epub 2020 Feb 21. Ageing Res Rev. 2020. PMID: 32092463 Review.
Cited by
- Auditory sensory gating in the neonatal ventral hippocampal lesion model of schizophrenia.
Vohs JL, Chambers RA, Krishnan GP, O'Donnell BF, Hetrick WP, Kaiser ST, Berg S, Morzorati SL. Vohs JL, et al. Neuropsychobiology. 2009;60(1):12-22. doi: 10.1159/000234813. Epub 2009 Aug 14. Neuropsychobiology. 2009. PMID: 19684419 Free PMC article. - Prepulse inhibition and genetic mouse models of schizophrenia.
Powell SB, Zhou X, Geyer MA. Powell SB, et al. Behav Brain Res. 2009 Dec 7;204(2):282-94. doi: 10.1016/j.bbr.2009.04.021. Epub 2009 May 4. Behav Brain Res. 2009. PMID: 19397931 Free PMC article. - Realistic expectations of prepulse inhibition in translational models for schizophrenia research.
Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Swerdlow NR, et al. Psychopharmacology (Berl). 2008 Aug;199(3):331-88. doi: 10.1007/s00213-008-1072-4. Epub 2008 Jun 21. Psychopharmacology (Berl). 2008. PMID: 18568339 Free PMC article. Review. - Sensory and sensorimotor gating-disruptive effects of apomorphine in Sprague Dawley and Long Evans rats.
Breier MR, Lewis B, Shoemaker JM, Light GA, Swerdlow NR. Breier MR, et al. Behav Brain Res. 2010 Apr 2;208(2):560-5. doi: 10.1016/j.bbr.2009.12.037. Epub 2010 Jan 18. Behav Brain Res. 2010. PMID: 20080128 Free PMC article. - In vitro and in vivo demonstration of risperidone implants in mice.
Rabin C, Liang Y, Ehrlichman RS, Budhian A, Metzger KL, Majewski-Tiedeken C, Winey KI, Siegel SJ. Rabin C, et al. Schizophr Res. 2008 Jan;98(1-3):66-78. doi: 10.1016/j.schres.2007.08.003. Epub 2007 Aug 31. Schizophr Res. 2008. PMID: 17765477 Free PMC article.
References
- Adams CE, Stevens KE. Inhibition of nitric oxide synthase disrupts inhibitory gating of auditory responses in rat hippocampus. J Pharmacol Exp Ther. 1998;287:760–765. - PubMed
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. - PubMed
- Adler LE, Rose G, Freedman R. Neurophysiological studies of sensory gating in rats: effects of amphetamine, phencyclidine, and haloperidol. Biol Psychiatry. 1986;21:787–798. - PubMed
- Bolino F, DiMichele V, DiCicco L, Manna V, Daneluzzo E, Casacchia M. Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenia. Biol Psychiatry. 1994;36:670–679. - PubMed
- Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res. 2004;126:203–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH01436/MH/NIMH NIH HHS/United States
- MH42228/MH/NIMH NIH HHS/United States
- R01 MH053484/MH/NIMH NIH HHS/United States
- R37 MH042228/MH/NIMH NIH HHS/United States
- R01 MH042228/MH/NIMH NIH HHS/United States
- MH53484/MH/NIMH NIH HHS/United States
- K02 MH001436/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials