Inhibition of Single Minded 2 gene expression mediates tumor-selective apoptosis and differentiation in human colon cancer cells - PubMed (original) (raw)
Inhibition of Single Minded 2 gene expression mediates tumor-selective apoptosis and differentiation in human colon cancer cells
Mireille J Aleman et al. Proc Natl Acad Sci U S A. 2005.
Abstract
A Down's syndrome associated gene, Single Minded 2 gene short form (SIM2-s), is specifically expressed in colon tumors but not in the normal colon. Antisense inhibition of SIM2-s in a RKO-derived colon carcinoma cell line causes growth inhibition, apoptosis, and inhibition of tumor growth in a nude mouse tumoriginicity model. The mechanism of cell death in tumor cells is unclear. In the present study, we investigated the pathways underlying apoptosis. Apoptosis was seen in a tumor cell-specific manner in RKO cells but not in normal renal epithelial cells, despite inhibition of SIM2-s expression in both of these cells by the antisense. Apoptosis was depended on WT p53 status and was caspase-dependent; it was inhibited by a pharmacological inhibitor of mitogen-activated protein kinase activity. Expression of a key stress response gene, growth arrest and DNA damage gene (GADD)45alpha, was up-regulated in antisense-treated tumor cells but not in normal cells. In an isogenic RKO cell line expressing stable antisense RNA to GADD45alpha, a significant protection of the antisense-induced apoptosis was seen. Whereas antisense-treated RKO cells did not undergo cell cycle arrest, several markers of differentiation were deregulated, including alkaline phosphatase activity, a marker of terminal differentiation. Protection of apoptosis and block of differentiation showed a correlation in the RKO model. Our results support the tumor cell-selective nature of SIM2-s gene function, provide a direct link between SIM2-s and differentiation, and may provide a model to identify SIM2-s targets.
Figures
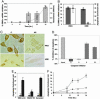
Fig. 1.
SIMS2-s in apoptosis. (A) SIM2-s mRNA inhibition precedes apoptosis. RKO cells were treated with either the control or SIM2-s antisense oligonucleotides and, at indicated times, SIM2-s mRNA (shaded) was analyzed by real-time quantitative RT-PCR and the level of apoptosis shown as enrichment (control oligos, ▴; antisense, -x-) was measured by ELISA. The average of two independent experiments + SEM is shown. (B) Induction of tumor cell-selective apoptosis. Percent of SIM2-s mRNA inhibition in 100 nM SIM2-s antisense-treated RKO or HRE cells for 24 h (shaded) compared with the level of apoptosis (enrichment) measured by ELISA in the same samples (unshaded). The average of two experiments + SEM is shown. (C) Inhibition of SIM2-s protein in tumor and normal cells by antisense. Immunohistochemistry analysis of RKO and HRE cells treated for 18 h with 100 nM of either the control (C) or the antisense (AS) oligonucleotides is shown. Arrows indicate nuclear stain (×320). (D) Caspase-dependent apoptosis. Percent inhibition of apoptosis in the presence of 20 μM either general caspase inhibitor (Z-VAD-FMK), caspase-2 inhibitor (Z-VDVAD-FMK), caspase-8 inhibitor (Z-IETD-FMK), caspase-9 inhibitor (Z-LEHD-FMK), or caspase-10 inhibitor (Z-AEVD-FMK) measured at 24 h in 100 nM SIM2-s antisense-treated RKO cells. The average of two independent experiments + SEM is shown. (E) MAPK signal is necessary for apoptosis. RKO cells were treated for 24 h with either control (gray) or SIM2-s antisense (shaded) in the presence or absence of 20 μM p38 MAPK inhibitor (SB202190) or 2.6 μM general tyrosine kinase inhibitor (Genistein). Apoptosis was measured by ELISA. The average of two independent experiments + SEM is shown. (F) Intact WT p53 is necessary for apoptosis. Level of apoptosis (enrichment) measured at 10, 14, 18, and 24 h in 100 nM SIM2-s control oligo-treated RKO (×) and RKO-E6 (•) cells vs. antisense treated RKO (▪) and RKO-E6 (▴) cells. The average of three independent experiments + SEM is shown.
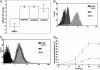
Fig. 2.
GADD45α as a critical mediator of apoptosis. (A) Up-regulation of GADD family members by SIM2-s inhibition. Expression of GADD family members (_GADD45_α, _GADD45_β, and GADD34) and SIM2-s was analyzed in the SIM2-s antisense-treated cells by real-time quantitative RT-PCR. The mRNA fold change in antisense-treated cells compared with the levels in the control oligonucleotide-treated cells is shown. The average of two independent experiments + SEM is shown. (B) Up-regulation of GADD45α expression in SIM2-s antisense-treated RKO cells. RKO cells were treated with 100 nM either control (C) or SIM2-s antisense (AS) for 18 h, and the cells were analyzed by FACS for GADD45α expression. A representative experiment of three independent experiments is shown. (C) Lack of GADD45α up-regulation in normal HRE cells. HRE cells were treated with 100 nM of either control (C) or SIM2-s antisense (AS) for 24 h, and the cells were analyzed by FACS for GADD45α expression. A representative experiment of two independent experiments is shown. (D) GADD45α null isogenic RKO protects from apoptosis induced by SIM2-s antisense. Level of apoptosis (enrichment) measured by ELISA at 10, 14, 18, and 24 h in 100 nM SIM2-s control oligo-treated RKO (×) and RKO-AS45.1 (•) cells vs. antisense-treated RKO (▪) and RKO-AS45.1 (▴) cells. The average of three independent experiments + SEM is shown.
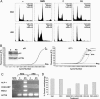
Fig. 3.
Cell-cycle-independent differentiation by SIM2-s. (A) Cell cycle profile of RKO and HRE cells treated at 14 or 24 h, respectively, either untreated (–) or treated with methylmethane sulfonate (25 μg/ml) or 100 nM of either the control oligonucleotide (C) or SIM2-s antisense oligonucleotide (AS). Fractions of cells in G1, S, and G2 phases are shown. A representative experiment of three independent experiments is shown. (B) Time-dependent deregulation of differentiation markers in antisense-treated RKO cells. Real-time RT-PCR analysis of the RKO cells treated with 100 nM either control (C) or SIM2-s antisense oligonucleotides (AS) for p21 (24 h) and ICT1 (10, 14, and 24 h) expression. (Inset) Western blot analysis for p21 protein. For ICT1 analysis, the control indicates 24-h control oligonucleotide treatment. Neg, template minus PCR control. A representative experiment of two independent experiments is shown. (C) Tumor-selective up-regulation of differentiation markers by SIM2-s antisense. RKO or HRE cells were treated with 100 nM either the control (C) or SIM2-s antisense oligonucleotides (AS) for 8 and 24 h, respectively, and the cells were analyzed by RT-PCR for expression of ALP1, MUC2, carcinoembryonic antigen 7, and actin genes. Neg, template minus PCR control. (D) ALP activity in SIM2-s antisense-treated RKO cells correlates with apoptosis. RKO cells were treated with sodium butyrate, NaB (3 mM), or 100 nM either control or SIM2-s antisense oligos (18 h) in the absence or presence of SB 202190 (20 μM) and Z-LEHD-FMK (20 μM), and ALP activity was measured. ALP activity is also shown for RKO-E6 and -AS45.1 cells after 18 h of antisense treatment. A representative experiment is shown.
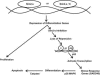
Fig. 4.
Proposed model for the role of SIM2-s in differentiation and apoptosis in tumor cells.
Similar articles
- Identification of Down's syndrome critical locus gene SIM2-s as a drug therapy target for solid tumors.
DeYoung MP, Tress M, Narayanan R. DeYoung MP, et al. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4760-5. doi: 10.1073/pnas.0831000100. Epub 2003 Apr 3. Proc Natl Acad Sci U S A. 2003. PMID: 12676991 Free PMC article. - Down's syndrome-associated single minded gene as a novel tumor marker.
Deyoung MP, Scheurle D, Damania H, Zylberberg C, Narayanan R. Deyoung MP, et al. Anticancer Res. 2002 Nov-Dec;22(6A):3149-57. Anticancer Res. 2002. PMID: 12530058 - Effects of p21cip1/waf1 overexpression on growth, apoptosis and differentiation in human colon carcinoma cells.
Izawa H, Yamamoto H, Damdinsuren B, Ikeda K, Tsujie M, Suzuki R, Kitani K, Seki Y, Hayashi T, Takemasa I, Ikeda M, Ohue M, Sekimoto M, Monden T, Monden M. Izawa H, et al. Int J Oncol. 2005 Jul;27(1):69-76. Int J Oncol. 2005. PMID: 15942645 - Id proteins in neural cancer.
Iavarone A, Lasorella A. Iavarone A, et al. Cancer Lett. 2004 Feb 20;204(2):189-96. doi: 10.1016/S0304-3835(03)00455-5. Cancer Lett. 2004. PMID: 15013218 Review. - Myeloid differentiation (MyD) primary response genes in hematopoiesis.
Liebermann DA, Hoffman B. Liebermann DA, et al. Oncogene. 2002 May 13;21(21):3391-402. doi: 10.1038/sj.onc.1205312. Oncogene. 2002. PMID: 12032777 Review.
Cited by
- SIM2: Its Prognostic Significance and Oncogenic Role in Endometrial Carcinoma.
Wei Y, Zhao X, Tang H, Ma J, Wang Y, Li L. Wei Y, et al. Onco Targets Ther. 2024 Jan 26;17:45-61. doi: 10.2147/OTT.S440788. eCollection 2024. Onco Targets Ther. 2024. PMID: 38292061 Free PMC article. - Ha-Ras transformation of MCF10A cells leads to repression of Singleminded-2s through NOTCH and C/EBPbeta.
Gustafson TL, Wellberg E, Laffin B, Schilling L, Metz RP, Zahnow CA, Porter WW. Gustafson TL, et al. Oncogene. 2009 Mar 26;28(12):1561-8. doi: 10.1038/onc.2008.497. Epub 2009 Jan 26. Oncogene. 2009. PMID: 19169276 Free PMC article. - Linoleic acid pathway disturbance contributing to potential cancerization of intrahepatic bile duct stones into intrahepatic cholangiocarcinoma.
Li J, Lu J, Lv S, Sun S, Liu C, Xu F, Sun H, Yang J, Wang X, Zhong X, Lu J. Li J, et al. BMC Gastroenterol. 2022 May 30;22(1):269. doi: 10.1186/s12876-022-02354-2. BMC Gastroenterol. 2022. PMID: 35637430 Free PMC article. - Methylated DNA markers for plasma detection of ovarian cancer: Discovery, validation, and clinical feasibility.
Marinelli LM, Kisiel JB, Slettedahl SW, Mahoney DW, Lemens MA, Shridhar V, Taylor WR, Staub JK, Cao X, Foote PH, Burger KN, Berger CK, O'Connell MC, Doering KA, Giakoumopoulos M, Berg H, Volkmann C, Solsrud A, Allawi HT, Kaiser M, Vaccaro AM, Albright Crawford C, Moehlenkamp C, Shea G, Deist MS, Schoolmeester JK, Kerr SE, Sherman ME, Bakkum-Gamez JN. Marinelli LM, et al. Gynecol Oncol. 2022 Jun;165(3):568-576. doi: 10.1016/j.ygyno.2022.03.018. Epub 2022 Mar 31. Gynecol Oncol. 2022. PMID: 35370009 Free PMC article. - A dock derived compound against laminin receptor (37 LR) exhibits anti-cancer properties in a prostate cancer cell line model.
Umbaugh CS, Diaz-Quiñones A, Neto MF, Shearer JJ, Figueiredo ML. Umbaugh CS, et al. Oncotarget. 2017 Dec 13;9(5):5958-5978. doi: 10.18632/oncotarget.23236. eCollection 2018 Jan 19. Oncotarget. 2017. PMID: 29464047 Free PMC article.
References
- Kola, I. & Hertzog, P. J. (1997) Hum. Mol. Genet. 6, 1713–1727. - PubMed
- Kola, I. & Hertzog, P. J. (1998) Curr. Opin. Genet. Dev. 8, 316–321. - PubMed
- Isaac, D. D. & Andrew, D. J. (1996) Genes Dev. 10, 103–117. - PubMed
- McGuire, J., Coumailleau, P., Whitelaw, M. L., Gustafsson, J. A. & Poellinger, L. (1995) J. Biol. Chem. 270, 31353–31357. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous