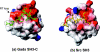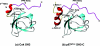Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implications for cellular signal transduction - PubMed (original) (raw)
Review
Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implications for cellular signal transduction
Shawn S-C Li. Biochem J. 2005.
Abstract
Protein-protein interactions occurring via the recognition of short peptide sequences by modular interaction domains play a central role in the assembly of signalling protein complexes and larger protein networks that regulate cellular behaviour. In addition to spatial and temporal factors, the specificity of signal transduction is intimately associated with the specificity of many co-operative, pairwise binding events upon which various pathways are built. Although protein interaction domains are usually identified via the recognition code, the consensus sequence motif, to which they selectively bind, they are highly versatile and play diverse roles in the cell. For example, a given interaction domain can bind to multiple sequences that exhibit no apparent identity, and, on the other hand, domains of the same class or different classes may favour a given consensus motif. This promiscuity in ligand selection is typified by the SH3 (Src homology 3) domain and several other interaction modules that commonly recognize proline-rich sequences. Furthermore, interaction domains are highly adaptable, a property that is essential for the evolution of novel pathways and modulation of signalling dynamics. The ability of certain interaction domains to perform multiple tasks, however, poses a challenge for the cell to control signalling specificity when cross-talk between pathways is undesired. Extensive structural and biochemical analysis of many interaction domains in recent years has started to shed light on the molecular basis underlying specific compared with diverse binding events that are mediated by interaction domains and the role affinity plays in affecting domain specificity and regulating cellular signal transduction.
Figures
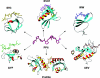
Figure 1. Structures of six PRDs in complex with their cognate ligands
All six PRDs recognize a common structural scaffold: the PPII helix. A PPII helix formed by a poly-
L
-Pro7 peptide is depicted in the centre to show the characteristics of the structure. Domains are portrayed in ribbon representations, while the bound peptides are shown in heavy atom traces. Structures were generated using MOLMOL [109]. The Protein Data Bank (PDB) accession codes for the structures are: 1QWE (SH3), 1EVH (EVH1), 1K9R (WW), 1L2Z (GYF), 1CJF (Profilin) and 1M4Q (UEV).
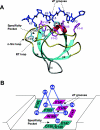
Figure 2. Structure of the Crk SH3-N domain in complex with a high-affinity peptide from C3G [62]
(A) The structure shown is based on PDB accession code 1CKB. The SH3 domain is depicted in ribbons with secondary-structural elements shown in different colours and labelled. The bound peptide, PPPALPPKKR, is shown in blue with side chains. Interface residues on the SH3 domain are shown in pink, for aromatic residues, and in light blue, for non-aromatic residues. The locations of the xP grooves and specificity pocket on the SH3 domain are identified by broken arrows. (B) A schematic representation of the same structure to highlight the characteristics of the ligand-binding surface on the SH3 domain such as the enrichment of aromatic residues. The same colouring scheme is used in (A) and (B) for purposes of comparison.
Figure 3. Novel mode of peptide recognition for the Gads SH3-C domain
The structure of the Gads SH3 domain (left) is depicted in surface representation to show the three distinct binding pockets for the SLP-76 peptide (in green). Areas of positive and negative charges are shown in blue and red respectively. Residue Glu275 in the RT loop of the SH3 domain, which encloses the second pocket, is labelled in white. For comparison, the structure of the c-Src SH3 domain in complex with a PxxP-containing peptide [38] is shown on the right. Note the differences in characteristics and size for the second and third pockets on the two SH3 domains.
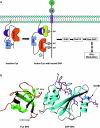
Figure 4. Diverse roles of SH3 domains in the phox proteins
A model for the translocation of the regulatory phox complex to the catalytic core of the NADPH oxidase through co-operative interactions mediated by SH3 and PX domains, leading to the assembly and activation of the holoenzyme (not shown in whole in the diagram). The p40–p47–p67_phox_ regulatory complex resides in the cytosol in the absence of a stimulating signal. The complex is kept together through high-affinity interactions involving SH3 and PB1 domains, as shown. The tandem SH3 domains of p47_phox_ were shown to interact with its polybasic region through a novel mode of ligand recognition (see text). This unique interaction renders p47_phox_ translocation-incompetent. Following a stimulus, p47_phox_ is phosphorylated on multiple serine residues in the polybasic region. Phosphorylation releases the auto-inhibition in p47_phox_, allowing it to associate with p22_phox_ through its SH3 domains. The PX domains in p47_phox_ and p40_phox_, capable of binding phosphoinositides, are believed to play an important role in the translocation of the regulatory phox complex by interacting with the membrane. aa, amino acids.
Figure 5. SAP, an adaptor composed of a single SH2 domain
(A) Signal transduction through the SLAM receptor is regulated by SAP. Fyn is kept in an auto-inhibited state in the absence of SAP. Recruitment of Fyn to the SLAM receptor by SAP leads to activation of the kinase and subsequent phosphorylation of SLAM on multiple tyrosine residues. Phosphorylated tyrosine residues serve as docking sites for the SH2 domain of SHIP (SH2-containing inositol phosphatase). Binding of SHIP to SLAM initiates a signalling cascade that ultimately leads to modulation of IFN-γ production in T cells. (B) Structure of a ternary complex of (Fyn SH3)–(SAP SH2)–SLAM peptide. The Fyn SH3 domain, in green, binds to the SAP SH2 domain, in cyan, via surface–surface association. The SAP SH2 domain engages the SLAM peptide (shown in magenta) on the opposite surface using a groove conserved for SH2 domains. The structure shown is Protein Data Bank accession code 1M27.
Figure 6. Interactions outside the PxxP motif augments affinity and specificity for SH3 domain
Shown on the left is the structure of the Csk SH3 domain–PEP peptide complex while on the right is the structure of the p67_phox_ SH3-C domain in complex with a 32-residue peptide derived from p47_phox_. SH3 domains in both complexes are depicted in cyan (with loops in grey). The structures of the bound peptides are labelled as follows: 310 and α-helices in red, PPII in magenta, and others in yellow.
Similar articles
- Formin binding proteins bear WWP/WW domains that bind proline-rich peptides and functionally resemble SH3 domains.
Chan DC, Bedford MT, Leder P. Chan DC, et al. EMBO J. 1996 Mar 1;15(5):1045-54. EMBO J. 1996. PMID: 8605874 Free PMC article. - Structural basis of the differential binding of the SH3 domains of Grb2 adaptor to the guanine nucleotide exchange factor Sos1.
McDonald CB, Seldeen KL, Deegan BJ, Farooq A. McDonald CB, et al. Arch Biochem Biophys. 2008 Nov 1;479(1):52-62. doi: 10.1016/j.abb.2008.08.012. Epub 2008 Aug 26. Arch Biochem Biophys. 2008. PMID: 18778683 - Elucidation of the binding preferences of peptide recognition modules: SH3 and PDZ domains.
Teyra J, Sidhu SS, Kim PM. Teyra J, et al. FEBS Lett. 2012 Aug 14;586(17):2631-7. doi: 10.1016/j.febslet.2012.05.043. Epub 2012 Jun 9. FEBS Lett. 2012. PMID: 22691579 Review.
Cited by
- Complex formation of EphB1/Nck/Caskin1 leads to tyrosine phosphorylation and structural changes of the Caskin1 SH3 domain.
Pesti S, Balázs A, Udupa R, Szabó B, Fekete A, Bőgel G, Buday L. Pesti S, et al. Cell Commun Signal. 2012 Nov 27;10(1):36. doi: 10.1186/1478-811X-10-36. Cell Commun Signal. 2012. PMID: 23181695 Free PMC article. - Structural distributions from single-molecule measurements as a tool for molecular mechanics.
Hanson JA, Brokaw J, Hayden CC, Chu JW, Yang H. Hanson JA, et al. Chem Phys. 2012 Mar 2;396:61-71. doi: 10.1016/j.chemphys.2011.06.014. Epub 2011 Jun 22. Chem Phys. 2012. PMID: 22661822 Free PMC article. - DLC1 SAM domain-binding peptides inhibit cancer cell growth and migration by inactivating RhoA.
Joshi R, Qin L, Cao X, Zhong S, Voss C, Min W, Li SSC. Joshi R, et al. J Biol Chem. 2020 Jan 10;295(2):645-656. doi: 10.1074/jbc.RA119.011929. Epub 2019 Dec 5. J Biol Chem. 2020. PMID: 31806702 Free PMC article. - Structural basis for ubiquitin recognition by SH3 domains.
He Y, Hicke L, Radhakrishnan I. He Y, et al. J Mol Biol. 2007 Oct 12;373(1):190-6. doi: 10.1016/j.jmb.2007.07.074. Epub 2007 Aug 17. J Mol Biol. 2007. PMID: 17765920 Free PMC article. - Role of Src signal transduction pathways in scatter factor-mediated cellular protection.
Fan S, Meng Q, Laterra JJ, Rosen EM. Fan S, et al. J Biol Chem. 2009 Mar 20;284(12):7561-77. doi: 10.1074/jbc.M807497200. Epub 2008 Dec 1. J Biol Chem. 2009. PMID: 19047046 Free PMC article.
References
- Pawson T., Nash P. Assembly of cell regulatory systems through protein interaction domains. Science. 2003;300:445–452. - PubMed
- Sudol M. From Src homology domains to other signaling modules: proposal of the ‘protein recognition code’. Oncogene. 1998;17:1469–1474. - PubMed
- Songyang Z. Recognition and regulation of primary-sequence motifs by signaling modular domains. Prog. Biophys. Mol. Biol. 1999;71:359–372. - PubMed
- Pawson T. Specificity in signal transduction: from phosphotyrosine–SH2 domain interactions to complex cellular systems. Cell. 2004;116:191–203. - PubMed
- Morgan M. J. Initial sequencing and analysis of the human genome. Nature (London) 2001;409:860–921. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous