NF-kappaB RelA phosphorylation regulates RelA acetylation - PubMed (original) (raw)
NF-kappaB RelA phosphorylation regulates RelA acetylation
Lin-Feng Chen et al. Mol Cell Biol. 2005 Sep.
Abstract
The nuclear functions of NF-kappaB p50/RelA heterodimers are regulated in part by posttranslational modifications of its RelA subunit, including phosphorylation and acetylation. Acetylation at lysines 218, 221, and 310 differentially regulates RelA's DNA binding activity, assembly with IkappaBalpha, and transcriptional activity. However, it remains unclear whether the acetylation is regulated or simply due to stimulus-coupled nuclear translocation of NF-kappaB. Using anti-acetylated lysine 310 RelA antibodies, we detected p300-mediated acetylation of RelA in vitro and in vivo after stimulation of cells with tumor necrosis factor alpha (TNF-alpha). Coexpression of catalytically inactive mutants of the catalytic subunit of protein kinase A/mitogen- and stress-activated kinase 1 or IKK1/IKK2, which phosphorylate RelA on serine 276 or serine 536, respectively, sharply inhibited RelA acetylation on lysine 310. Furthermore, phosphorylation of RelA on serine 276 or serine 536 increased assembly of phospho-RelA with p300, which enhanced acetylation on lysine 310. Reconstitution of RelA-deficient murine embryonic fibroblasts with RelA S276A or RelA S536A decreased TNF-alpha-induced acetylation of lysine 310 and expression of the endogenous NF-kappaB-responsive E-selectin gene. These findings indicate that the acetylation of RelA at lysine 310 is importantly regulated by prior phosphorylation of serines 276 and 536. Such phosphorylated and acetylated forms of RelA display enhanced transcriptional activity.
Figures
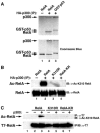
FIG. 1.
RelA is acetylated on lysine 310 by p300 in vitro. (A) p300 immunoprecipitated from 293T cells acetylates RelA in vitro. 293T cells were cotransfected with HA-p300 expression vector DNA. p300 was immunoprecipitated from the cells and incubated with 1 μg of recombinant RelA (lane 2) or GST-p53 (lane 3) in an in vitro acetylation assay. Autoradiograms showing the acetylation of RelA and p53 and the autoacetylation of p300 are shown in the top panel. Levels of p300, RelA, and GST-p53 detected by Coomassie blue staining are shown in the bottom panel. (B) Anti-acetylated lysine 310 RelA antibodies specifically recognize acetylated lysine 310. Recombinant proteins (1 μg) of wild-type RelA, K310R, or RelA-KR were incubated with immunoprecipitated HA-p300 in an in vitro acetylation assay. Reaction products were separated by SDS-polyacrylamide gel electrophoresis, and acetylation (Ac) was detected by immunoblotting with anti-acetylated lysine 310 antibodies (top). Levels of RelA, RelA-K310, and RelA-KR are shown in the bottom panels. (C). p300 acetylates RelA at lysine 310 in vivo. 293T cells were cotransfected with expression vector DNA encoding wild-type RelA, K310R, or RelA-KR (0.5 μg) and p300 (2 μg). Acetylation was detected by immunoblotting of anti-T7 immunoprecipitates with anti-acetylated lysine 310 antibodies (top). Levels of T7-RelA and the various RelA mutants are shown in the bottom panel. IB, immunoblotting; IP, immunoprecipitation.
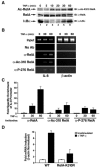
FIG. 2.
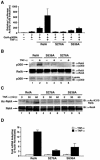
Stimulus-coupled acetylation of endogenous RelA on lysine 310 in vivo. (A) TNF-α induces lysine 310 acetylation of RelA in vivo. 293T cells were stimulated with TNF-α (20 ng/ml) for the indicated periods of time. Whole-cell extracts were prepared, and the acetylation (Ac) of endogenous RelA was detected by immunoblotting (IB) with anti-acetylated lysine-310 antibodies (top). Levels of RelA and IκBα are shown in the middle and bottom panels, respectively. (B) TNF-α induces the binding of lysine 310-acetylated RelA, and phospho-276 RelA binds to the κB enhancer of the IL-8 gene. HeLa cells were treated with TNF-α for the indicated periods. ChIP assays were performed using anti-RelA (α-RelA), anti-acetylated lysine 310 (α-Ac-310), or anti-phosphorylated serine 276 antibodies or in the absence of antibodies (No Ab) and probed for the IL-8 promoter sequences spanning the κB binding sites (left panels) or for nonspecific control β-actin DNA (right panels). (C) Quantification of ChIP binding using RT-PCR and cyber green. Samples presented as described above (B) were analyzed as described in Materials and Methods. Data represent the average of two independent experiments. (D) TNF-α-induced expression of E-selectin, an endogenous NF-κB target gene, is impaired in RelA-deficient MEFs reconstituted with the RelA-K310R mutant. RelA-deficient MEFs reconstituted with either wild-type (WT) RelA or RelA-K310R were stimulated with TNF-α for 0 or 6 h. Total RNA from cells was isolated, and expression levels of E-selectin mRNA were measured by quantitative real-time PCR. Results represent the averages of two independent experiments.
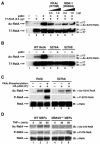
FIG. 3.
Phosphorylation of serine 276 is critical for the acetylation of RelA. (A) Enzymatically inactive forms of PKAc and MSK-1 inhibit acetylation (Ac) of RelA at lysine 310. 293T cells were cotransfected with the expression plasmid DNA encoding T7-RelA, p300, and graded doses of PKAc(K47M) or MSK-1(D656A), as indicated. Acetylation of RelA was detected by immunoblotting (IB) of the anti-T7 (α-T7) immunoprecipitates (IP) with anti-acetylated lysine 310 antibodies (top). Levels of T7-RelA present in each of the lysates are shown in the bottom panel. (B) Alanine or glutamic acid substitutions at serine 276 block the acetylation of RelA at lysine 310. 293T cells were transfected with wild-type (WT) RelA, S276A, or S276E expression vectors (0.5 μg) together with p300 (2 μg). Levels of acetylation of each protein were detected by immuno-blotting of anti-T7 immunoprecipitates with anti-acetylated lysine 310 antibodies. Levels of RelA, S276A, and S276E are shown in the bottom panel. (C) Phosphorylation of RelA at serine 276 by PKAc enhances the acetylation of RelA in vitro. Recombinant proteins (1 μg) of wild-type or S276A RelA were phosphorylated with recombinant PKAc (0.5 μl) (Promega) in an in vitro kinase assay. Reactions were then incubated with p300 immunoprecipitates in an in vitro acetylation assay. Levels of acetylation of RelA were detected by immunoblotting with anti-acetylated lysine 310 antibodies. Levels of RelA in each reaction are shown in the bottom panel. (D) Failure of TNF-α to induce acetylation of RelA on lysine 310 in MSK-1/MSK-2-deficient MEFs. MEFs from MSK-1/MSK-2-deficient mice were stimulated with TNF-α for the indicated time periods. Acetylation and phosphorylation levels of RelA were analyzed by immunoblotting with anti-acetylated lysine 310 antibodies (top) or with anti-phosphorylated serine 276 antibodies (α-P-276) (middle). The levels of RelA present in each of the cell extracts are also shown in the bottom panel.
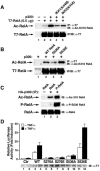
FIG. 4.
Phosphorylation of RelA at serine 536 facilitates the acetylation of RelA on lysine 310. (A) Kinase-deficient mutants of IKK1 and IKK2 inhibit the acetylation (Ac) of RelA at lysine 310. 293T cells were cotransfected with the indicated expression plasmid DNA encoding T7-RelA (0.5 μg), p300 (2 μg), IKK1(K47M) (0.5 μg), and IKK2(K47A) (0.5 μg). Acetylation of RelA was detected by immunoblotting (IB) of anti-T7 (α-T7) immunoprecipitates (IP) with anti-acetylated lysine 310 (α-Ac-K310) antibodies (top). Levels of T7-RelA present in each of the lysates are shown in the bottom panel. (B) Alanine or glutamic acid substitutions at serine 536 impair or enhance, respectively, the acetylation of RelA at lysine 310. 293T cells were transfected with wild-type (WT) RelA, S536A, or S536E expression vectors (0.5 μg) together with p300 (2 μg). Levels of acetylation on each protein were detected by immunoblotting of anti-T7 immunoprecipitates with anti-acetylated lysine 310 antibodies. Levels of RelA, S536A, and S536E are shown in the bottom panel. (C) Phosphorylation of RelA at serine 536 by IKK2 enhances the acetylation of RelA in vitro. Recombinant RelA (1 μg) was phosphorylated with recombinant IKK2 (0.5 μl) (P-RelA) in an in vitro kinase assay. Reactions were then incubated with p300 immunoprecipitates in an in vitro acetylation assay as described in the legend to Fig. 1A. Levels of acetylation of RelA were detected by immunoblotting with anti-acetylated lysine 310 antibodies. Levels of RelA in each reaction are shown in the bottom panel. (D) RelA-deficient MEFs were transfected with E-selectin-luciferase reporter plasmid DNA (0.5 μg) and the various S276A, S276E, S536A, and S536E mutants of RelA (0.1 μg). After 36 h, cells were treated with or without TNF-α (20 ng/ml) for 6 h. Luciferase activity was measured as described previously (4). Expression levels of RelA and various RelA mutants in each transfection are shown in the bottom panel. Results represent the averages of three independent experiments ± standard deviations.
FIG. 5.
Phosphorylation of RelA at serines 276 and 536 enhances the association of RelA with p300. (A) Alanine substitution of serines 276 and 536 impairs RelA interaction with p300 in a mammalian one-hybrid assay. RelA-deficient MEFs were transfected with expression plasmids (1 ng) encoding RelA, S276A, or S536A and GAL4-p300N (50 ng) together with a GAL4 enhancer-luciferase reporter (0.1 μg) (pFR-luc). After 24 h, the cells were stimulated with TNF-α for 5 h. Luciferase activity was measured as previously described (4). Results represent the averages of three independent experiments ± standard deviations. (B) TNF-α stimulates the interaction of wild-type RelA, but not the S276A and S536A mutants, with p300. MEFs stably expressing the wild type or the S276A or S536A mutant were stimulated with TNF-α for 60 min. Whole-cell lysates were then immunoprecipitated (IP) with anti-RelA (α-RelA) antibodies (catalog number F-6; Santa Cruz Biotechnology) followed by immunoblotting (IB) with anti-p300 (α-p300) (catalog number SC-584; Santa Cruz Biotechnology). Levels of p300 in the lysates and immunoprecipitated RelA are shown in the bottom two panels. (C) TNF-α induced acetylation of lysine 310 in reconstituted cells with wild-type RelA. MEFs reconstituted with wild-type, S276A, and S536A RelA were stimulated with TNF-α (20 ng/ml) for the indicated periods of time, whole-cell extracts were prepared, and the acetylation of RelA (Ac-RelA) was detected by immunoblotting with anti-acetylated lysine 310 (α-Ac-K310) antibodies (top). Levels of RelA are shown in the bottom panel. (D) TNF-α-induced activation of the endogenous E-selectin gene is impaired in RelA-deficient MEFs reconstituted with the RelA S276A or S536A mutant. RelA-deficient MEFs reconstituted with either wild-type RelA, RelA S276A, or RelA S536A were stimulated with TNF-α for 0 or 6 h. Total RNA from cells was isolated, and expression levels of E-selectin mRNA were measured by quantitative real-time PCR. Results represent the averages of two independent experiments.
FIG. 6.
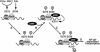
Schematic model for the role of phosphorylation of serines 276 and 536 in the regulation of RelA acetylation. PKAc- or MSK-1-mediated phosphorylation of serine 276 or IKK-mediated phosphorylation of serine 536 (A) leads to more effective recruitment of p300 (B), which in turn mediates the acetylation (Ac) of lysine 310 (C). Phosphorylated and acetylated forms of RelA then exhibit potential transcription of NF-κB-dependent genes (D).
Similar articles
- Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation.
Duran A, Diaz-Meco MT, Moscat J. Duran A, et al. EMBO J. 2003 Aug 1;22(15):3910-8. doi: 10.1093/emboj/cdg370. EMBO J. 2003. PMID: 12881425 Free PMC article. - Enhancement of nuclear factor-kappa B acetylation by coactivator p300 and HIV-1 Tat proteins.
Furia B, Deng L, Wu K, Baylor S, Kehn K, Li H, Donnelly R, Coleman T, Kashanchi F. Furia B, et al. J Biol Chem. 2002 Feb 15;277(7):4973-80. doi: 10.1074/jbc.M107848200. Epub 2001 Dec 5. J Biol Chem. 2002. PMID: 11739381 - Insufficient p65 phosphorylation at S536 specifically contributes to the lack of NF-kappaB activation and transformation in resistant JB6 cells.
Hu J, Nakano H, Sakurai H, Colburn NH. Hu J, et al. Carcinogenesis. 2004 Oct;25(10):1991-2003. doi: 10.1093/carcin/bgh198. Epub 2004 Jun 10. Carcinogenesis. 2004. PMID: 15192014 - Regulation of NF-kappaB action by reversible acetylation.
Greene WC, Chen LF. Greene WC, et al. Novartis Found Symp. 2004;259:208-17; discussion 218-25. Novartis Found Symp. 2004. PMID: 15171256 Review. - Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation.
Chen LF, Greene WC. Chen LF, et al. J Mol Med (Berl). 2003 Sep;81(9):549-57. doi: 10.1007/s00109-003-0469-0. Epub 2003 Aug 15. J Mol Med (Berl). 2003. PMID: 12920522 Review.
Cited by
- Human Bocavirus NS1 and NS1-70 Proteins Inhibit TNF-α-Mediated Activation of NF-κB by targeting p65.
Liu Q, Zhang Z, Zheng Z, Zheng C, Liu Y, Hu Q, Ke X, Wang H. Liu Q, et al. Sci Rep. 2016 Jun 22;6:28481. doi: 10.1038/srep28481. Sci Rep. 2016. PMID: 27329558 Free PMC article. - Transcription Factors in Cartilage Homeostasis and Osteoarthritis.
Neefjes M, van Caam APM, van der Kraan PM. Neefjes M, et al. Biology (Basel). 2020 Sep 14;9(9):290. doi: 10.3390/biology9090290. Biology (Basel). 2020. PMID: 32937960 Free PMC article. Review. - Mitogen- and stress-activated kinase 1 (MSK1) regulates cigarette smoke-induced histone modifications on NF-κB-dependent genes.
Sundar IK, Chung S, Hwang JW, Lapek JD Jr, Bulger M, Friedman AE, Yao H, Davie JR, Rahman I. Sundar IK, et al. PLoS One. 2012;7(2):e31378. doi: 10.1371/journal.pone.0031378. Epub 2012 Feb 1. PLoS One. 2012. PMID: 22312446 Free PMC article. - Quantitative acetylome analysis reveals the roles of SIRT1 in regulating diverse substrates and cellular pathways.
Chen Y, Zhao W, Yang JS, Cheng Z, Luo H, Lu Z, Tan M, Gu W, Zhao Y. Chen Y, et al. Mol Cell Proteomics. 2012 Oct;11(10):1048-62. doi: 10.1074/mcp.M112.019547. Epub 2012 Jul 23. Mol Cell Proteomics. 2012. PMID: 22826441 Free PMC article. - Breast cancer metastasis suppressor 1 functions as a corepressor by enhancing histone deacetylase 1-mediated deacetylation of RelA/p65 and promoting apoptosis.
Liu Y, Smith PW, Jones DR. Liu Y, et al. Mol Cell Biol. 2006 Dec;26(23):8683-96. doi: 10.1128/MCB.00940-06. Epub 2006 Sep 25. Mol Cell Biol. 2006. PMID: 17000776 Free PMC article.
References
- Baldwin, A. S., Jr. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. - PubMed
- Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. - PubMed
- Bohuslav, J., L. F. Chen, H. Kwon, Y. Mu, and W. C. Greene. 2004. p53 Induces NF-κB activation by an IκB kinase-independent mechanism involving RSK1 phosphorylation of p65. J. Biol. Chem. 279:26115-26125. - PubMed
- Chen, L. F., W. Fischle, E. Verdin, and W. C. Greene. 2001. Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293:1653-1657. - PubMed
- Chen, L. F., and W. C. Greene. 2004. Shaping the nuclear action of NF-κB. Nat. Rev. Mol. Cell Biol. 5:392-401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous