The cleavage of microphthalmia-associated transcription factor, MITF, by caspases plays an essential role in melanocyte and melanoma cell apoptosis - PubMed (original) (raw)
The cleavage of microphthalmia-associated transcription factor, MITF, by caspases plays an essential role in melanocyte and melanoma cell apoptosis
Lionel Larribere et al. Genes Dev. 2005.
Abstract
Microphthalmia-associated transcription factor (MITF) M-form is a melanocyte-specific transcription factor that plays a key role in melanocyte development, survival, and differentiation. Here, we identified MITF as a new substrate of caspases and we characterized the cleavage site after Asp 345 in the C-terminal domain. We show that expression of a noncleavable form of MITF renders melanoma cells resistant to apoptotic stimuli, and we found that the C-terminal fragment generated upon caspase cleavage is endowed with a proapoptotic activity that sensitizes melanoma cells to death signals. The proapoptotic function gained by MITF following its processing by caspases provides a tissue-restricted means to modulate death in melanocyte and melanoma cells.
Figures
Figure 1.
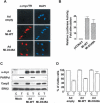
MITF is proteolytically processed by caspases during apoptosis. Primary human melanocytes (A), human WM9 melanomas (B), or human MeWo melanomas (C) were incubated with TRAIL (T) (R&D Systems) for the times and at the concentrations indicated. Cell lysates were analyzed by Western blot with antibodies that recognize MITF, procaspase 3 (Casp 3), and total ERK2. (D) Human MeWo melanomas were preincubated with 50 μM Ac-DEVD-fmk (Alexis, Qbiogen, SA) for 24 h and then were exposed to 50 ng/mL TRAIL for 2 h. Western blot assays were performed using procaspase 3, MITF, and total ERK2 antibodies. (E) In vitro-translated MITF was incubated with recombinant caspases 3, 6, or 7 (BD Biosciences, Pharmingen) in the presence or absence of 10 μM acetyl DEVD-fmk, and was then separated by electrophoresis and analyzed by autoradiography. (F) In vitro-translated wild-type MITF (WT) or MITF mutants (D309A, D334A, D345A, D380A) were exposed to recombinant caspase 3. (G) A293 cells were transiently transfected with empty pCDNA3 or pCDNA3 encoding wild-type MITF (Mi-WT), uncleavable MITF (Mi-D345A), or MITF N-terminal 1-345 (Mi-NT), and then exposed to 50 ng/mL TRAIL for 2.5 h. Protein lysates were analyzed by immunoblotting with specific MITF, ERK2, and procaspase 3 antibodies. (H) In vitro-translated MITF, TFEB, and TFE3 were incubated with recombinant caspase 3, then separated by electrophoresis and analyzed by autoradiography.
Figure 2.
A noncleavable mutant of MITF prevents TRAIL-induced apoptosis of melanoma cells. (A) MeWo melanoma cells that were infected for 48 h with an empty adenovirus (Ad-empty) or adenoviruses encoding Mi-WT (Ad Mi-WT) or Mi-D345A (Ad Mi-D345A) were analyzed by immunofluorescence with anti-myc and secondary-coupled Texas-Red (TR) antibodies and DAPI staining. (B) Luciferase assay using B16 cells transfected with Tyrosinase promoter reporter (200 ng), pCMV-βgalactosidase (50 ng), and the expression vectors (50 ng) encoding wild-type (Mi-WT) or uncleavable (Mi-D345A) MITF. (C) WM9 melanomas were infected with Ad-empty, Ad Mi-WT, or Ad Mi-D345A for 48 h at MOI (multiplicity of infection) 20 and then cells were exposed to 50 ng/mL TRAIL for 2 h. Western blots were carried out with anti-myc, cleaved PARP (PARPcl), or procaspase 3 (Casp3)-specific antibodies. (D) Survival of WM9 melanomas treated as in C was assessed by the XTT dye reduction assay.
Figure 3.
siRNA-mediated down-regulation of MITF does not promote death. (A) Cell lysates of human MeWo melanoma cells treated with si-MITF or si-cont for 48 h were probed with MITF-, procaspase 3 (Casp3)-, Bcl2-, and ERK2-specific antibodies. (B) MeWo melanoma cells, transfected with si-MITF or si-LUC, were left untreated or were exposed to TRAIL (50 ng/mL) for 4 h. Adherent cells were collected and counted using an automatic cell counter or solubilized to perform Western blotting using MITF or procaspase 3 antibodies.
Figure 4.
MITF C terminus induces melanoma cell death. (A) B16 cells were transiently transfected with pCMV-βGal (50 ng) and indicated expression vectors (50 ng) for wild-type (WT), uncleavable (Mi-D345A), or N-terminal (Mi-NT) or C-terminal (Mi-CT) MITF mutants and then assayed for β-galactosidase activity. (B) Immunofluorescence studies with anti-myc and secondary-coupled Texas-Red (TR) antibodies and DAPI staining of MeWo melanomas infected with empty adenovirus (Ad-empty) or encoding MITF C-terminal (Ad Mi-CT) adenoviruses at MOI 20 for 48 h. Western blotting with anti-myc, procaspase 3 (Casp3), cleaved PARP (PARPcl), or total ERK2 antibodies (C) and phase-contrast microscopy photos of MeWo cells infected with Ad-empty or Ad Mi-CT (D) at MOI 20 for 72 h. (E) MeWo melanoma cells infected with Ad-empty or Ad Mi-CT at MOI 20 for 72 h or exposed to TRAIL for 15 h were stained with the FITC-conjugated active-caspase-3 antibody Apoptosis kit. Fluorescence was measured by using the FL1 channel of a FACScan.
Similar articles
- Hypoxia-inducible factor 1{alpha} is a new target of microphthalmia-associated transcription factor (MITF) in melanoma cells.
Buscà R, Berra E, Gaggioli C, Khaled M, Bille K, Marchetti B, Thyss R, Fitsialos G, Larribère L, Bertolotto C, Virolle T, Barbry P, Pouysségur J, Ponzio G, Ballotti R. Buscà R, et al. J Cell Biol. 2005 Jul 4;170(1):49-59. doi: 10.1083/jcb.200501067. Epub 2005 Jun 27. J Cell Biol. 2005. PMID: 15983061 Free PMC article. - The melanocyte-specific isoform of the microphthalmia transcription factor affects the phenotype of human melanoma.
Selzer E, Wacheck V, Lucas T, Heere-Ress E, Wu M, Weilbaecher KN, Schlegel W, Valent P, Wrba F, Pehamberger H, Fisher D, Jansen B. Selzer E, et al. Cancer Res. 2002 Apr 1;62(7):2098-103. Cancer Res. 2002. PMID: 11929831 - Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression.
Carreira S, Goodall J, Aksan I, La Rocca SA, Galibert MD, Denat L, Larue L, Goding CR. Carreira S, et al. Nature. 2005 Feb 17;433(7027):764-9. doi: 10.1038/nature03269. Nature. 2005. PMID: 15716956 - Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival.
Widlund HR, Fisher DE. Widlund HR, et al. Oncogene. 2003 May 19;22(20):3035-41. doi: 10.1038/sj.onc.1206443. Oncogene. 2003. PMID: 12789278 Review. - [Malignant melanoma and the role of the paradoxal protein Microphthalmia transcription factor].
Denat L, Larue L. Denat L, et al. Bull Cancer. 2007 Jan;94(1):81-92. Bull Cancer. 2007. PMID: 17237008 Review. French.
Cited by
- Coordinated regulation of chromatophore differentiation and melanogenesis during the ontogeny of skin pigmentation of Solea senegalensis (Kaup, 1858).
Darias MJ, Andree KB, Boglino A, Fernández I, Estévez A, Gisbert E. Darias MJ, et al. PLoS One. 2013 May 9;8(5):e63005. doi: 10.1371/journal.pone.0063005. Print 2013. PLoS One. 2013. PMID: 23671650 Free PMC article. - MITF-the first 25 years.
Goding CR, Arnheiter H. Goding CR, et al. Genes Dev. 2019 Aug 1;33(15-16):983-1007. doi: 10.1101/gad.324657.119. Epub 2019 May 23. Genes Dev. 2019. PMID: 31123060 Free PMC article. Review. - Pro-survival role of MITF in melanoma.
Hartman ML, Czyz M. Hartman ML, et al. J Invest Dermatol. 2015 Feb;135(2):352-358. doi: 10.1038/jid.2014.319. Epub 2014 Aug 21. J Invest Dermatol. 2015. PMID: 25142731 Review. - MITF in melanoma: mechanisms behind its expression and activity.
Hartman ML, Czyz M. Hartman ML, et al. Cell Mol Life Sci. 2015 Apr;72(7):1249-60. doi: 10.1007/s00018-014-1791-0. Epub 2014 Nov 30. Cell Mol Life Sci. 2015. PMID: 25433395 Free PMC article. Review. - A role for tyrosinase-related protein 1 in 4-tert-butylphenol-induced toxicity in melanocytes: Implications for vitiligo.
Manga P, Sheyn D, Yang F, Sarangarajan R, Boissy RE. Manga P, et al. Am J Pathol. 2006 Nov;169(5):1652-62. doi: 10.2353/ajpath.2006.050769. Am J Pathol. 2006. PMID: 17071589 Free PMC article.
References
- Bertolotto C., Busca, R., Abbe, P., Bille, K., Aberdam, E., Ortonne, J.P., and Ballotti, R. 1998b. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol. Cell. Biol. 18: 694-702. - PMC - PubMed
- Carreira S., Goodall, J., Aksan, I., La Rocca, S.A., Galibert, M.D., Denat, L., Larue, L., and Goding, C.R. 2005. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 433: 764-769. - PubMed
- Ciccaglione A.R., Marcantonio, C., Tritarelli, E., Equestre, M., Magurano, F., Costantino, A., Nicoletti, L., and Rapicetta, M. 2004. The transmembrane domain of hepatitis C virus E1 glycoprotein induces cell death. Virus Res. 104: 1-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical