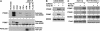ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage - PubMed (original) (raw)
Comparative Study
. 2005 Oct 5;24(19):3411-22.
doi: 10.1038/sj.emboj.7600812. Epub 2005 Sep 15.
Affiliations
- PMID: 16163388
- PMCID: PMC1276172
- DOI: 10.1038/sj.emboj.7600812
Comparative Study
ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage
Lihong Chen et al. EMBO J. 2005.
Abstract
The p53 tumor suppressor is activated after DNA damage to maintain genomic stability and prevent transformation. Rapid activation of p53 by ionizing radiation is dependent on signaling by the ATM kinase. MDM2 and MDMX are important p53 regulators and logical targets for stress signals. We found that DNA damage induces ATM-dependent phosphorylation and degradation of MDMX. Phosphorylated MDMX is selectively bound and degraded by MDM2 preceding p53 accumulation and activation. Reduction of MDMX level by RNAi enhances p53 response to DNA damage. Loss of ATM prevents MDMX degradation and p53 stabilization after DNA damage. Phosphorylation of MDMX on S342, S367, and S403 were detected by mass spectrometric analysis, with the first two sites confirmed by phosphopeptide-specific antibodies. Mutation of MDMX on S342, S367, and S403 each confers partial resistance to MDM2-mediated ubiquitination and degradation. Phosphorylation of S342 and S367 in vivo require the Chk2 kinase. Chk2 also stimulates MDMX ubiquitination and degradation by MDM2. Therefore, the E3 ligase activity of MDM2 is redirected to MDMX after DNA damage and contributes to p53 activation.
Figures
Figure 1
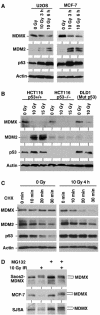
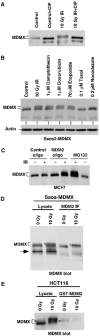
DNA damage induces degradation of MDMX. (A, B) Cell lines with different p53 mutation status were treated with IR for 3–6 h and analyzed for expression levels of indicated markers by Western blot. (C) MCF7 cells were treated with 10 Gy IR and cultured for 4 h. Cyclohexamide (CHX, 100 μg/ml) was added to inhibit protein synthesis and samples were analyzed for the rates of MDMX, MDM2, and p53 degradation. (D) Saos2 cells stably transfected with MDMX expression plasmid (Saos2-MDMX) or MCF7 and SJSA cells expressing endogenous MDMX were treated with IR and MG132 for 4 h and analyzed for MDMX expression by gradient gel electrophoresis and Western blot. IR induces the appearance of a slow migrating form of MDMX.
Figure 2
Phosphorylation of MDMX promotes degradation by MDM2. (A) Saos2-MDMX cells were treated with IR and immunoprecipitated using MDMX monoclonal antibody 8C6. The immunoprecipitate was incubated with CIP and changes in MDMX mobility was determined by gradient gel Western blot. (B) Saos2-MDMX cells were treated with indicated agents for 6 h and MDMX was analyzed by gradient gel Western blot. (C) MCF7 cells were treated with 200 nM MDM2 antisense oligonucleotide or control oligonucleotide for 24 h by lipofectin-mediated transfection and then irradiated with 10 Gy IR for 6 h. MDMX was detected by Western blot. Loading was empirically adjusted to compensate for the loss of MDMX and to detect the phosphorylated population. (D) Saos2-MDMX cells were tested for binding efficiencies of MDM2 to different forms of MDMX by MDM2 IP and MDMX Western blot after irradiation in the presence of MG132. The arrow indicates a background band in the whole-cell lysate crossreacting with the rabbit-anti-MDMX serum. (E) Glutathione-agarose beads loaded with GST-MDM2 were incubated with extract of control and irradiated HCT116 cells (treated with MG132). The captured MDMX were analyzed by gradient gel Western blot. The phosphorylated MDMX preferentially bound by MDM2 or GST-MDM2 was marked by *.
Figure 3
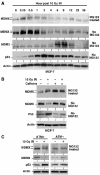
MDMX phosphorylation after DNA damage requires ATM activity. (A) MCF7 cells were irradiated with 10 Gy IR and samples collected at different time points were analyzed by gradient gel Western blot for the indicated markers. (B) MCF-7 cells were irradiated at 10 Gy in the presence of 10 mM caffeine and MG132 and MDMX phosphorylation was determined after 6 h by Western blot in the top panel (loading was empirically adjusted to show phosphorylated forms rather than degradation). Cells in the second panel were treated without MG132 and MDMX degradation was determined by Western blot. (C) Human skin fibroblasts that are wild type or ATM-deficient were irradiated in the presence or absence of MG132 for 4 h. MDMX phosphorylation and degradation were determined by gradient gel electrophoresis and Western blot.
Figure 4
Knockdown of MDMX enhances p53 response to DNA damage. (A) U2OS cells were transfected with control or MDMX siRNA for 48 h, treated with 10 Gy IR, and analyzed for p53 activation at the indicated time points. (B) U2OS cells transfected with control or MDMX siRNA for 48 h were treated with 10 Gy IR, and starting at the indicated time points labeled for 2 h with BrdU. The levels of BrdU incorporation were measured by an ELISA assay (_n_=4).
Figure 5
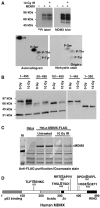
Mapping of phosphorylation sites on MDMX. (A) H1299 cells transiently transfected with MDMX were treated with 10 Gy IR and immediately labeled for 4 h with 32P orthophosphate. MDMX was immunoprecipitated, run on SDS–PAGE, transferred to nylon membrane and detected by autoradiography and Western blot. Radiolabeled MDMX bands were hydrolysed by HCl and analyzed by 2-D electrophoresis. Autoexposure of radiolabeled MDMX residues and ninhydrin stain of phospho-amino-acid standards on the same cellulose plate were shown. (B) Saos2 cells stably transfected with MDMX deletion mutants were treated with radiation and MDMX mobility was determined by gradient gel Western blot. (C) HeLa cells stably transfected with FLAG-tagged MDMX were treated with 10 Gy IR for 6 h, immunoprecipitated using M2-agarose beads, fractionated on SDS–PAGE, and stained by Coomassie blue. Entire MDMX bands from control and irradiated cells were excised and subjected to mass spectrometric analysis. (D) Diagram of MDMX and relative positions of phosphorylation sites identified by mass spectrometry on irradiated MDMX. Bold letter indicate the modified serine.
Figure 6
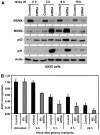
Effects of MDMX phosphorylation site mutations. (A) H1299 cells stably transfected with MDMX point mutants were irradiated at 10 Gy and MDMX mobility shift was analyzed after 6 h by Western blot. The 342A, 367A, and 403A mutants significantly reduced MDMX mobility shift. (B) MDMX mutant plasmids (4 μg) were cotransfected with 0, 0.5, 1, 2 μg of MDM2 plasmid into H1299 cells and MDMX expression levels were determined by Western blot 48 h after transfection. The same cell lysate were also analyzed by MDM2 IP and MDMX Western blot to detect MDM2–MDMX binding. The 367A mutant retained MDM2 binding function but was resistant to degradation by MDM2. (C) MDMX mutants were cotransfected with His6-ubiquitin and MDM2 into H1299 cells. Ubiquitinated MDMX were detected by Western blot after purification using Ni-NTA beads 48 h after transfection. (D) U2OS cells stably infected with retrovirus vector (LXSN) expressing MDMX or MDMX-342A/367A/403A triple mutant (MDMX-3A) was treated with 10 Gy gamma irradiation for 4 h and analyzed for expression of indicated markers of p53 activation by Western blot.
Figure 7
Detection of MDMX phosphorylation by peptide-specific antibodies. (A, B) MCF-7 cells treated with 10 Gy IR for 4 h in the presence of MG132 were immunoprecipitated using 8C6 antibody and probed with anti-phospho S342 or S367 peptide antibodies. His6-MDMX expressed in E. coli was used as a control for crossreactivity to nonphosphorylated MDMX. The same membranes were striped and probed using MDMX polyclonal antibody to determine the level of total MDMX. (C) U2OS cells stably transfected with MDMX were irradiated with 10 Gy IR and MDMX was immunoprecipitated after 2 h and probed with PS342 and PS367 antibodies. The presence of basal level S342 phosphorylation on unirradiated MDMX was confirmed by treatment with CIP. The membranes were reprobed with anti-MDMX polyclonal antibody to confirm loading. (D) U2OS stably transfected with MDMX point mutants were treated with 10 Gy IR for 2 h and analyzed by MDMX IP and PS342 and PS367 Western blot.
Figure 8
Phosphorylation of MDMX by Chk1 and Chk2 kinases. (A) In vitro phosphorylation of S342 and S367 by Chk1 and Chk2. Recombinant His6-MDMX and His6-p53 purified from E. coli were incubated with indicated kinases in the presence of ATP. The reaction products were analyzed by Western blot using PS342, PS467, and p53PS15 antibodies. (B) U2OS stably transfected with MDMX was treated with gamma irradiation for 2 h in the presence of 10 mM caffeine and analyzed by MDMX IP and PS342 and PS367 Western blot. (C) Chk2-null HCT116 cells were treated with 10 Gy gamma irradiation for 4 h in the presence of MG132, and analyzed for MDMX phosphorylation by IP-Western blot.
Figure 9
Regulation of MDMX degradation by Chk2. (A) Chk2-null HCT116 cells were transiently transfected with MDMX, FLAG-Chk2, or inactive FLAG-Chk2-A347 mutant. Cells were treated with 10 Gy gamma irradiation for 2 h in the presence of MG132 and analyzed for MDMX S367 phosphorylation by IP-Western blot. The Chk2 blot used a Chk2-specific antibody that detects both endogenous and transfected Chk2. (B) Chk2-null HCT116 cells were transfected with indicated plasmids and analyzed for MDMX ubiquitination and degradation after 48 h. (C) Chk2-null HCT116 cells were treated with 5 Gy gamma irradiation and analyzed for MDMX degradation, induction of MDM2, and stabilization of p53 at indicated time points. (D) DNA damage activates p53 through multiple ATM-dependent mechanisms.
Similar articles
- Differential roles of ATM- and Chk2-mediated phosphorylations of Hdmx in response to DNA damage.
Pereg Y, Lam S, Teunisse A, Biton S, Meulmeester E, Mittelman L, Buscemi G, Okamoto K, Taya Y, Shiloh Y, Jochemsen AG. Pereg Y, et al. Mol Cell Biol. 2006 Sep;26(18):6819-31. doi: 10.1128/MCB.00562-06. Mol Cell Biol. 2006. PMID: 16943424 Free PMC article. - Phosphorylation of Hdmx mediates its Hdm2- and ATM-dependent degradation in response to DNA damage.
Pereg Y, Shkedy D, de Graaf P, Meulmeester E, Edelson-Averbukh M, Salek M, Biton S, Teunisse AF, Lehmann WD, Jochemsen AG, Shiloh Y. Pereg Y, et al. Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):5056-61. doi: 10.1073/pnas.0408595102. Epub 2005 Mar 23. Proc Natl Acad Sci U S A. 2005. PMID: 15788536 Free PMC article. - DNA damage-induced phosphorylation of MdmX at serine 367 activates p53 by targeting MdmX for Mdm2-dependent degradation.
Okamoto K, Kashima K, Pereg Y, Ishida M, Yamazaki S, Nota A, Teunisse A, Migliorini D, Kitabayashi I, Marine JC, Prives C, Shiloh Y, Jochemsen AG, Taya Y. Okamoto K, et al. Mol Cell Biol. 2005 Nov;25(21):9608-20. doi: 10.1128/MCB.25.21.9608-9620.2005. Mol Cell Biol. 2005. PMID: 16227609 Free PMC article. - How to activate p53.
Caspari T. Caspari T. Curr Biol. 2000 Apr 20;10(8):R315-7. doi: 10.1016/s0960-9822(00)00439-5. Curr Biol. 2000. PMID: 10801407 Review. - ATM-mediated phosphorylations inhibit Mdmx/Mdm2 stabilization by HAUSP in favor of p53 activation.
Meulmeester E, Pereg Y, Shiloh Y, Jochemsen AG. Meulmeester E, et al. Cell Cycle. 2005 Sep;4(9):1166-70. doi: 10.4161/cc.4.9.1981. Epub 2005 Sep 29. Cell Cycle. 2005. PMID: 16082221 Review.
Cited by
- Antiproliferative factor signaling and interstitial cystitis/painful bladder syndrome.
Kim J, Freeman MR. Kim J, et al. Int Neurourol J. 2011 Dec;15(4):184-91. doi: 10.5213/inj.2011.15.4.184. Epub 2011 Dec 31. Int Neurourol J. 2011. PMID: 22259731 Free PMC article. - Secondary interaction between MDMX and p53 core domain inhibits p53 DNA binding.
Wei X, Wu S, Song T, Chen L, Gao M, Borcherds W, Daughdrill GW, Chen J. Wei X, et al. Proc Natl Acad Sci U S A. 2016 May 10;113(19):E2558-63. doi: 10.1073/pnas.1603838113. Epub 2016 Apr 25. Proc Natl Acad Sci U S A. 2016. PMID: 27114532 Free PMC article. - Predicted functions of MdmX in fine-tuning the response of p53 to DNA damage.
Kim S, Aladjem MI, McFadden GB, Kohn KW. Kim S, et al. PLoS Comput Biol. 2010 Feb 5;6(2):e1000665. doi: 10.1371/journal.pcbi.1000665. PLoS Comput Biol. 2010. PMID: 20174603 Free PMC article. - Intra molecular interactions in the regulation of p53 pathway.
Chen J. Chen J. Transl Cancer Res. 2016 Dec;5(6):639-649. doi: 10.21037/tcr.2016.09.23. Transl Cancer Res. 2016. PMID: 30613472 Free PMC article. - 14-3-3Gamma inhibition of MDMX-mediated p21 turnover independent of p53.
Lee JH, Lu H. Lee JH, et al. J Biol Chem. 2011 Feb 18;286(7):5136-42. doi: 10.1074/jbc.M110.190470. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148311 Free PMC article.
References
- Ahn J, Urist M, Prives C (2003) Questioning the role of checkpoint kinase 2 in the p53 DNA damage response. J Biol Chem 278: 20480–20489 - PubMed
- Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421: 499–506 - PubMed
- Banin S, Moyal L, Shieh S-Y, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y (1998) Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281: 1674–1677 - PubMed
- Bartek J, Lukas J. (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–449 - PubMed
- Chao C, Hergenhahn M, Kaeser MD, Wu Z, Saito S, Iggo R, Hollstein M, Appella E, Xu Y (2003) Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem 278: 41028–41033 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous