Mutually exclusive subsets of BH3-only proteins are activated by the p53 and c-Jun N-terminal kinase/c-Jun signaling pathways during cortical neuron apoptosis induced by arsenite - PubMed (original) (raw)
Mutually exclusive subsets of BH3-only proteins are activated by the p53 and c-Jun N-terminal kinase/c-Jun signaling pathways during cortical neuron apoptosis induced by arsenite
Hon Kit Wong et al. Mol Cell Biol. 2005 Oct.
Abstract
The c-Jun N-terminal protein kinase (JNK)/c-Jun and p53 pathways form distinct death-signaling modules in neurons that culminate in Bax-dependent apoptosis. To investigate whether this signaling autonomy is due to recruitment of particular BH3-only proteins, we searched for a toxic signal that would activate both pathways in the same set of neurons. We show that arsenite activates both the JNK/c-Jun and p53 pathways in cortical neurons, which together account for >95% of apoptosis, as determined by using the mixed-lineage kinase (JNK/c-Jun) pathway inhibitor CEP11004 and p53-null mice. Despite the coexistence of both pathways in at least 30% of the population, Bim mRNA and protein expression was increased only by the JNK/c-Jun signaling pathway, whereas Noxa and Puma mRNA and Puma protein expression was entirely JNK/c-Jun independent. About 50% of Puma/Noxa expression was p53 dependent, with the remaining signal being independent of both pathways and possibly facilitated by arsenite-induced reduction in P-Akt. However, functionally, Puma was predominant in mediating Bax-dependent apoptosis, as evidenced by the fact that more than 90% of apoptosis was prevented in Puma-null neurons, although Bim was still upregulated, while Bim- and Noxa-null neurons died similarly to wild-type neurons. Thus, the p53 and JNK/c-Jun pathways can activate mutually exclusive subclasses of BH3-only proteins in the same set of neurons. However, other factors besides expression may determine which BH3-only proteins mediate apoptosis.
Figures
FIG. 1.
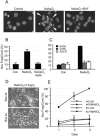
Arsenite causes Bax- and caspase-dependent apoptosis of mouse cortical neurons that requires macromolecular synthesis. (A) Micrographs showing nuclear morphology of Hoechst 33342-stained 7DIV cortical neurons that were either left untreated (left) or treated with 6 μM arsenite (NaAsO2) for 24 h (center and right) in the absence (center) or presence (right) of 100 μM BAF. Arrowheads indicate nuclear fragmentation and condensation. (B) Apoptosis was quantified by determining the proportion of neurons containing condensed and fragmented nuclei as indicated in panel A. (C) Cortical neurons were treated with 6 μM arsenite alone (black) or together with 1 μg/ml CHX (gray) or 1 μg/ml act-D (white) for 24 h, after which apoptosis was determined as for panel B. (D) Phase-contrast micrographs showing morphology of wt or Bax-null cortical neurons 3 days after addition of 6 μM arsenite. (E) The percentage of apoptosis was quantified as for panel B. con, control. Error bars, standard errors of the means from three independent experiments.
FIG. 2.
Arsenite induces Puma, Noxa, Bim, and Bid gene expression in 6DIV cortical neurons. (A) Changes in Bcl-2 family gene expression in neurons induced by treatment with 6 μM arsenite in the absence or presence of 100 μM BAF after 20 h, or in the presence of 1 μg/ml act-D (which reduces RNA synthesis by ∼93%) alone or with arsenite to investigate mRNA stability. GAPDH was amplified together with each sample as an internal control. Representative panels of ethidium bromide-stained gels after inversion of the digitized image are shown. (B) Mean change in gene expression ± 95% confidence interval from an analysis of four to six replicates. The intensity of each band was normalized to its respective internal control and divided by the normalized value for the untreated control. Error bars show only the upper limit of the 95% confidence interval for clarity, Black bars, change in the presence of arsenite without or with BAF present. Gray bars, change in the presence of act-D without or with arsenite present.
FIG. 3.
Amounts of Bax, Bim, and Puma protein are increased in the mitochondrial fraction of neurons after treatment with arsenite. 6DIV cortical neurons were treated with 6 μM arsenite and 100 μM BAF, and lysates were fractionated at the times indicated into an HM fraction and the corresponding cytosol (cyto). Proteins were separated by SDS-polyacrylamide gel electrophoresis and immunoblotted with the indicated antibodies. (A) The same proportion of volume from each cyto and HM fraction was loaded at each time point to assay for protein translocation. In order to match the volume of the HM fraction, the cyto fraction had to be concentrated, causing high background in some of the COX(IV) lanes in the cyto fraction. con, control. (B) A constant amount of protein (30 μg) of the HM and cyto fractions was loaded per lane to compare total amounts of Puma and cytochrome c (Cyt c) in the two fractions. (C) Band intensities of Bax and Bim expressed in the cyto and HM fractions in panel A were measured at each time point, from which the proportion of Bax or Bim in the HM fraction was calculated (mean ± SD from three independent experiments). Since significant amounts of Puma were found only in the HM fraction, band intensities of Puma and cytochrome c expressed in the HM fraction were measured and normalized to those of COX(IV) (mean ± SD from three to four independent experiments). *, P < 0.05 for comparison to time zero by Student's t test.
FIG. 4.
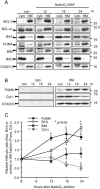
Arsenite activates c-Jun- and p53-dependent apoptosis and reduces P-Akt(Ser473). (A) 6DIV cortical neurons were treated with 6 μM arsenite for 24 h in the absence (−) or presence (CEP) of 5 μM CEP11004, after which proteins were separated by SDS-polyacrylamide gel electrophoresis and immunoblotted for P-JNK, JNK, P-c-Jun(Ser63), p53, P-Akt(Ser473), Akt, and ERK as a loading control. Note that in the absence of CEP11004, P-c-Jun(Ser63) and p53 expression was increased whereas P-Akt(Ser473) expression was decreased; CEP11004 prevented the phosphorylation of P-JNK and P-c-Jun(Ser63) proteins and the decrease in P-Akt(Ser473) without affecting p53 expression. (B) Neurons were fixed after 24 h of treatment, immunostained for p53, and visualized using an Alexa 488-conjugated secondary antibody. Nuclei were stained with Hoechst 33342. (C) The percentage of neurons with p53-positive nuclei is shown as a function of time after arsenite addition (mean ± range from two independent experiments). (D) Neurons from p53 wt or p53-null mice were treated with 6 μM arsenite in the absence or presence of 5 μM CEP11004 for 24 h, after which apoptosis was determined in unfixed cultures by scoring the percentage of nuclei stained with Hoechst 33342 that had condensed/fragmented DNA. Nuclei stained with PI were excluded. P < 0.01; means ± standard errors of the means from three independent experiments are shown. (E) p53 wt or p53 ko neurons were treated with 6 μM arsenite and either 100 μM BAF, 5 μM CEP11004, or 20 μM SP600125. Note that both the latter compounds blocked c-Jun phosphorylation but only CEP11004 blocked JNK phosphorylation. Neither compound affected p53 expression. (F) Comparison of efficacies of CEP11004 and SP600125. Neurons were treated as for panel D, except that either 5 μM CEP11004 or 20 μM SP600125 was used as a JNK inhibitor (JNKinh). (G) Images of neurons treated with 6 μM arsenite for 20 h, fixed, and costained with an anti-P-H2A.X(Ser139) antibody (with an Alexa 488-conjugated secondary antibody) and an anti-P-c-Jun(Ser63) antibody (with a Cy3-conjugated secondary antibody). Untreated controls were stained side by side. Arrows point to neurons showing costaining for P-c-Jun and P-H2A.X. (H) Reduction in P-Akt(Ser473) in neurons treated with 6 μM arsenite for 24 h. The band intensity of P-Akt was quantified, normalized to total Akt, and divided by the ratio of P-Akt to total Akt in untreated samples after 24 h. Data, from blots similar to those shown in panel A, are means ± standard errors of the means from seven independent experiments.
FIG. 5.
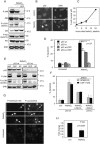
Induction of Puma and Noxa mRNA and Puma protein expression is dependent on p53 but is not dependent on the JNK/c-Jun pathway. (A) Neurons were either left untreated (control) or treated with 6 μM arsenite in the presence of 100 μM BAF. Proteins extracted at time zero or after 12 or 24 h were resolved by SDS-polyacrylamide gel electrophoresis, immunoblotted, and probed for p53, Puma, Mdm2, and total ERK as a loading control. (B) Relative amounts of the respective proteins (means ± ranges from two independent experiments). (C) 6DIV neurons from wt or p53-null animals were either left untreated or treated with 6 μM arsenite in the absence or presence of 5 μM CEP11004 for 24 h. Noxa, Puma, p53, and GAPDH mRNA expression was detected by RT-PCR. Note the significant decrease in expression of Puma and Noxa mRNA and the lack of effect of CEP11004 on the inducibility of Noxa and Puma mRNA. (D) Proteins from neurons treated as for panel A were separated by SDS-polyacrylamide gel electrophoresis and immunoblotted for p53, Mdm2, Puma, JNK, P-JNK, P-c-Jun(Ser63), and tubulin as a loading control. Note that a lack of p53 does not entirely inhibit the induction of Puma expression, though absolute levels are similar to those obtained from untreated p53 wt neurons.
FIG. 6.
Induction of Bim and Bid by arsenite is quenched by CEP11004, but induction is not affected by a lack of p53. (A) Cortical neurons from p53 wt or p53-null mice were treated for 24 h with 6 μM arsenite in the absence or presence of 5 μM CEP11004 (CEP) or 20 μM SP600125 (SP). In one sample, 100 μM BAF was added to ensure that survival was at least equivalent to that obtained with CEP. Proteins were extracted, resolved by SDS-polyacrylamide gel electrophoresis, and immunoblotted for Bim and tubulin (Tub.) as a loading control. Note the lack of effect on induction of Bim in p53-null neurons, though basal levels of expression relative to tubulin are higher. (B) Bid mRNA expression analyzed by RT-PCR. Note the suppression of arsenite-induced Bid expression by CEP11004 but the lack of inhibition of arsenite-induced Bid mRNA expression in p53-null neurons. Results of one of two representative experiments for wt neurons are shown. (C) Representative immunoblot (one of three independent experiments) of neurons treated as for panel A except that in addition, 100 μM BAF was added to one of the arsenite-treated cultures. The blot was probed for full length Bid (flBid; exposure, 15 s) and tBid (exposure, 15 min). Note the reduction of flBid in neurons undergoing apoptosis, the prevention of Bid degradation in neurons cotreated with BAF, and the correlation between the amount of reduction in tBid and the amount of apoptosis in the population.
FIG. 7.
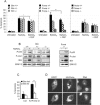
Puma is the dominant proapoptotic mediator of arsenite-induced, Bax-dependent death. (A) Percent apoptosis of arsenite-treated 6DIV cortical neurons derived from individual cortices of Bim, Puma, or Noxa littermates produced from heterozygote matings (three mice were wt for Bim, three for Puma, and four for Noxa; four mice were heterozygous for Bim, five for Puma, and four for Noxa; there were three Bim ko, four Puma ko, and four Noxa ko mice). Apoptosis was scored after 20 h. Note the lack of inhibition of death in _Bim_- or _Noxa_-null neurons compared to 90% inhibition in _Puma_-null neurons. (B) Blots show that neurons of all three Bim genotypes and of the Puma ko genotype express P-p53(Ser18) and P-c-Jun(Ser63), while Bim upregulation (which was 2-fold in wt neurons and 2.1-fold in Puma ko neurons) is Bim gene dosage dependent and remains prominent in Puma ko neurons. (C) Neurons were cultured from seven animals, four Bax−/− and three Bax+/− cortices. Cultures were infected in suspension with 40 PFU of Ad HA-Puma per neuron, and neurons coexpressing EGFP under the control of a separate CMV promoter in the same construct were scored for apoptosis after 20 h. Control (Con) neurons were uninfected. (D) Images of wt neurons infected with 40 PFU of Ad-Puma per neuron. Neurons were maintained in the presence of 100 μM BAF after infection to prevent apoptosis, fixed after 20 h, and immunostained for HA-Puma using an anti-HA antibody. DNA was stained with Hoechst 33342. Note the punctate localization of HA-Puma in cytoplasmic compartments compared to the diffuse staining of EGFP.
Similar articles
- The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in β-amyloid-induced neuron death.
Akhter R, Sanphui P, Das H, Saha P, Biswas SC. Akhter R, et al. J Neurochem. 2015 Sep;134(6):1091-103. doi: 10.1111/jnc.13128. Epub 2015 Apr 28. J Neurochem. 2015. PMID: 25891762 - The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons.
Wyttenbach A, Tolkovsky AM. Wyttenbach A, et al. J Neurochem. 2006 Mar;96(5):1213-26. doi: 10.1111/j.1471-4159.2005.03676.x. J Neurochem. 2006. PMID: 16478523 - Activation of Jun N-terminal kinase is a mediator of vincristine-induced apoptosis of melanoma cells.
Zhu BK, Wang P, Zhang XD, Jiang CC, Chen LH, Avery-Kiejda KA, Watts R, Hersey P. Zhu BK, et al. Anticancer Drugs. 2008 Feb;19(2):189-200. doi: 10.1097/CAD.0b013e3282f3138a. Anticancer Drugs. 2008. PMID: 18176116 - PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis.
Chipuk JE, Green DR. Chipuk JE, et al. Cell Cycle. 2009 Sep 1;8(17):2692-6. doi: 10.4161/cc.8.17.9412. Epub 2009 Sep 2. Cell Cycle. 2009. PMID: 19652530 Free PMC article. Review. - c-Jun N-terminal kinase signaling in cellular senescence.
Deng Y, Adam V, Nepovimova E, Heger Z, Valko M, Wu Q, Wei W, Kuca K. Deng Y, et al. Arch Toxicol. 2023 Aug;97(8):2089-2109. doi: 10.1007/s00204-023-03540-1. Epub 2023 Jun 19. Arch Toxicol. 2023. PMID: 37335314 Review.
Cited by
- BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death.
Akhtar RS, Geng Y, Klocke BJ, Latham CB, Villunger A, Michalak EM, Strasser A, Carroll SL, Roth KA. Akhtar RS, et al. J Neurosci. 2006 Jul 5;26(27):7257-64. doi: 10.1523/JNEUROSCI.0196-06.2006. J Neurosci. 2006. PMID: 16822983 Free PMC article. - Multiple mechanisms repress N-Bak mRNA translation in the healthy and apoptotic neurons.
Jakobson M, Jakobson M, Llano O, Palgi J, Arumäe U. Jakobson M, et al. Cell Death Dis. 2013 Aug 22;4(8):e777. doi: 10.1038/cddis.2013.297. Cell Death Dis. 2013. PMID: 23969856 Free PMC article. - Apoptotic cell death regulation in neurons.
Hollville E, Romero SE, Deshmukh M. Hollville E, et al. FEBS J. 2019 Sep;286(17):3276-3298. doi: 10.1111/febs.14970. Epub 2019 Jul 12. FEBS J. 2019. PMID: 31230407 Free PMC article. Review. - Implication of TAp73 in the p53-independent pathway of Puma induction and Puma-dependent apoptosis in primary cortical neurons.
Fricker M, Papadia S, Hardingham GE, Tolkovsky AM. Fricker M, et al. J Neurochem. 2010 Aug;114(3):772-83. doi: 10.1111/j.1471-4159.2010.06804.x. Epub 2010 May 8. J Neurochem. 2010. PMID: 20477944 Free PMC article. - BDNF stimulation of protein synthesis in cortical neurons requires the MAP kinase-interacting kinase MNK1.
Genheden M, Kenney JW, Johnston HE, Manousopoulou A, Garbis SD, Proud CG. Genheden M, et al. J Neurosci. 2015 Jan 21;35(3):972-84. doi: 10.1523/JNEUROSCI.2641-14.2015. J Neurosci. 2015. PMID: 25609615 Free PMC article.
References
- Akhtar, R. S., J. M. Ness, and K. A. Roth. 2004. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim. Biophys. Acta 1644:189-203. - PubMed
- Annis, M. G., J. A. Yethon, B. Leber, and D. W. Andrews. 2004. There is more to life and death than mitochondria: Bcl-2 proteins at the endoplasmic reticulum. Biochim. Biophys. Acta 1644:115-123. - PubMed
- Bar-Peled, O., M. Knudson, S. J. Korsmeyer, and J. D. Rothstein. 1999. Motor neuron degeneration is attenuated in bax-deficient neurons in vitro. J. Neurosci. Res. 55:542-556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous