Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension - PubMed (original) (raw)
Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension
Grazyna Kwapiszewska et al. Respir Res. 2005.
Abstract
Background: Chronic hypoxia influences gene expression in the lung resulting in pulmonary hypertension and vascular remodelling. For specific investigation of the vascular compartment, laser-microdissection of intrapulmonary arteries was combined with array profiling.
Methods and results: Analysis was performed on mice subjected to 1, 7 and 21 days of hypoxia (FiO2 = 0.1) using nylon filters (1176 spots). Changes in the expression of 29, 38, and 42 genes were observed at day 1, 7, and 21, respectively. Genes were grouped into 5 different classes based on their time course of response. Gene regulation obtained by array analysis was confirmed by real-time PCR. Additionally, the expression of the growth mediators PDGF-B, TGF-beta, TSP-1, SRF, FGF-2, TIE-2 receptor, and VEGF-R1 were determined by real-time PCR. At day 1, transcription modulators and ion-related proteins were predominantly regulated. However, at day 7 and 21 differential expression of matrix producing and degrading genes was observed, indicating ongoing structural alterations. Among the 21 genes upregulated at day 1, 15 genes were identified carrying potential hypoxia response elements (HREs) for hypoxia-induced transcription factors. Three differentially expressed genes (S100A4, CD36 and FKBP1a) were examined by immunohistochemistry confirming the regulation on protein level. While FKBP1a was restricted to the vessel adventitia, S100A4 and CD36 were localised in the vascular tunica media.
Conclusion: Laser-microdissection and array profiling has revealed several new genes involved in lung vascular remodelling in response to hypoxia. Immunohistochemistry confirmed regulation of three proteins and specified their localisation in vascular smooth muscle cells and fibroblasts indicating involvement of different cells types in the remodelling process. The approach allows deeper insight into hypoxic regulatory pathways specifically in the vascular compartment of this complex organ.
Figures
Figure 1
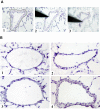
Intrapulmonary arteries. A) laser-microdissection of small intrapulmonary arteries. 1) The laser cuts along the outer side of the tunica adventitia. 2) A sterile needle is used to isolate the vessel. 3) Needle with adherent vessel is lifted and transferred afterwards to a reaction tube. Magnification × 200. B) Representative intrapulmonary arteries during the process of vascular remodelling. 1) Under normoxic conditions. 2) At day 1 of hypoxia. 3) At day 7 of hypoxia. Smooth muscle cell layer causes vascular thickening. 4) At day 21 of hypoxia. Magnification × 200.
Figure 2
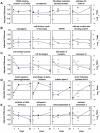
Comparison of array based time course of expression to that obtained by real-time RT-PCR (red: array; blue: TaqMan). A) Matrix γ-carboxyglutamate protein. B) Procollagen 3 α1. C) Prosaposin.
Figure 3
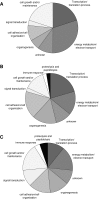
Gene classification according to biological processes. Significantly regulated genes were grouped according to their biological processes from NCBI, Gene Ontology, AmiGo. A) 1 day hypoxia, B) 7days hypoxia, C) 21days hypoxia.
Figure 4
Putative HIF-responsive elements (HRE) of the genes upregulated at day 1. Twenty genes were screened for the presence of the consensus sequence "BACGTSSK" 5000 bp up- and downstream the coding sequence. Aldolase C, a known HIF-responsive gene, was excluded. Fifteen genes were found carrying one or more putative HREs.
Figure 5
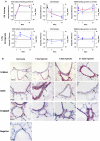
Regulation of S100A4, CD36 and FKBP1a on mRNA and protein level. A) Comparison of regulation between laser-microdissected arteries and lung homogenate from 1, 7, and 21 days of hypoxia exposure. (Red: array; blue: TaqMan). B) Immunohistochemical staining of S100A4, CD36 and FKBP1a in the mouse lung.
Figure 6
Immunolocalisation of S100A4. A) S100A4 protein (left panel) co-localises with alpha-smooth muscle actin (right panel). B) Small vessels (marked by arrows) are negative for S100A4 under normoxia (left panel) however stain positive for S100A4 after 21 days of hypoxia.
Figure 7
Classification of genes with similar regulation pattern. Four representatives each are given. A) Continuous upregulation at day 1, 7, and 21. B) Continuous downregulation at day 1, 7, and 21. C) Primarily upregulated, afterwards decrease under normoxic level (= downregulation). D) Primarily downregulated, afterwards increase over normoxic level (= upregulation). E) Primarily not regulated, afterwards up- or downregulated ("late response").
Similar articles
- Increased S100A4 expression in the vasculature of human COPD lungs and murine model of smoke-induced emphysema.
Reimann S, Fink L, Wilhelm J, Hoffmann J, Bednorz M, Seimetz M, Dessureault I, Troesser R, Ghanim B, Klepetko W, Seeger W, Weissmann N, Kwapiszewska G. Reimann S, et al. Respir Res. 2015 Oct 20;16:127. doi: 10.1186/s12931-015-0284-5. Respir Res. 2015. PMID: 26483185 Free PMC article. - Clinical perspective of hypoxia-mediated pulmonary hypertension.
Preston IR. Preston IR. Antioxid Redox Signal. 2007 Jun;9(6):711-21. doi: 10.1089/ars.2007.1587. Antioxid Redox Signal. 2007. PMID: 17511586 Review. - Cellular responses to hypoxia in the pulmonary circulation.
Welsh DJ, Peacock AJ. Welsh DJ, et al. High Alt Med Biol. 2013 Jun;14(2):111-6. doi: 10.1089/ham.2013.1016. High Alt Med Biol. 2013. PMID: 23795730 Review.
Cited by
- Molecular mechanisms of hypoxia-inducible factor-induced pulmonary arterial smooth muscle cell alterations in pulmonary hypertension.
Veith C, Schermuly RT, Brandes RP, Weissmann N. Veith C, et al. J Physiol. 2016 Mar 1;594(5):1167-77. doi: 10.1113/JP270689. Epub 2015 Sep 30. J Physiol. 2016. PMID: 26228924 Free PMC article. Review. - Gene expression analysis of a murine model with pulmonary vascular remodeling compared to end-stage IPAH lungs.
Shimodaira K, Okubo Y, Ochiai E, Nakayama H, Katano H, Wakayama M, Shinozaki M, Ishiwatari T, Sasai D, Tochigi N, Nemoto T, Saji T, Kamei K, Shibuya K. Shimodaira K, et al. Respir Res. 2012 Nov 17;13(1):103. doi: 10.1186/1465-9921-13-103. Respir Res. 2012. PMID: 23157700 Free PMC article. - Urine Proteomics for Noninvasive Monitoring of Biomarkers in Bronchopulmonary Dysplasia.
Ahmed S, Odumade OA, van Zalm P, Smolen KK, Fujimura K, Muntel J, Rotunno MS, Winston AB, Steen JA, Parad RB, Van Marter LJ, Kourembanas S, Steen H. Ahmed S, et al. Neonatology. 2022;119(2):193-203. doi: 10.1159/000520680. Epub 2022 Jan 24. Neonatology. 2022. PMID: 35073553 Free PMC article. - Targeting non-malignant disorders with tyrosine kinase inhibitors.
Grimminger F, Schermuly RT, Ghofrani HA. Grimminger F, et al. Nat Rev Drug Discov. 2010 Dec;9(12):956-70. doi: 10.1038/nrd3297. Nat Rev Drug Discov. 2010. PMID: 21119733 Review. - Mechanisms of disease: pulmonary arterial hypertension.
Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Schermuly RT, et al. Nat Rev Cardiol. 2011 Jun 21;8(8):443-55. doi: 10.1038/nrcardio.2011.87. Nat Rev Cardiol. 2011. PMID: 21691314 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous