Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor - PubMed (original) (raw)
Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor
Tracie Devries-Seimon et al. J Cell Biol. 2005.
Abstract
Macrophage death in advanced atherosclerosis promotes necrosis and plaque destabilization. A likely cause of macrophage death is accumulation of free cholesterol (FC) in the ER, leading to activation of the unfolded protein response (UPR) and C/EBP homologous protein (CHOP)-induced apoptosis. Here we show that p38 MAPK signaling is necessary for CHOP induction and apoptosis. Additionally, two other signaling pathways must cooperate with p38-CHOP to effect apoptosis. One involves the type A scavenger receptor (SRA). As evidence, FC loading by non-SRA mechanisms activates p38 and CHOP, but not apoptosis unless the SRA is engaged. The other pathway involves c-Jun NH2-terminal kinase (JNK)2, which is activated by cholesterol trafficking to the ER, but is independent of CHOP. Thus, FC-induced apoptosis requires cholesterol trafficking to the ER, which triggers p38-CHOP and JNK2, and engagement of the SRA. These findings have important implications for understanding how the UPR, MAPKs, and the SRA might conspire to cause macrophage death, lesional necrosis, and plaque destabilization in advanced atherosclerotic lesions.
Figures
Figure 1.
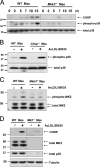
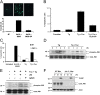
Activation of Mkk3/p38 MAPK is necessary for CHOP induction in FC-loaded macrophages. (A) WT and Mkk3 − / − macrophages (Mφs) were FC-loaded for 0, 2, 5, 7, 10, and 15 h using 100 μg/ml ac-LDL plus the ACAT inhibitor 58035. Whole cell lysates were prepared as described under “Materials and methods” and immunoblotted for CHOP (top panel), activated phospho-Thr180/Tyr182 p38 MAPK (phospho-p38, middle panel), and total p38 (bottom panel). Lines indicate areas of the same gel that were spliced together. (B) WT and Chop − / − macrophages were left untreated (−) or were FC-loaded (+) for 5 h, and lysates were immunoblotted for phospho-p38 and total p38. (C) WT and Mkk3 − / − macrophages were left untreated (−) or were FC-loaded for 7 h (+). In some experiments, WT cells were treated with ac-LDL alone, which showed only minimal MK2 phosphorylation (not depicted). Whole cell lysates were immunoblotted for activated phospho-Thr334 MK2 (phospho-MK2, top panel) and total MK2 (bottom panel). (D) WT and Mk2 − / − macrophages were left untreated (−) or were FC-loaded for 7 h (+). Whole cell lysates were immunoblotted for CHOP (top panel), total MK2 (second panel), total p38 (third panel), or tubulin as a loading control (bottom panel). Lines indicate areas of the same gel that were spliced together.
Figure 2.
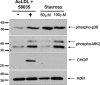
Staurosporine activates p38 MAPK and MK2, but does not induce CHOP in macrophages. Macrophages were left untreated (−), FC loaded for 6 h (+) using ac-LDL plus 58035, or treated for 6 h with 50 or 100 μM staurosporine. Immunoblot analysis was performed for phospho-p38 or phospho-MK2 (first and second panels), CHOP (third panel), and actin (bottom panel).
Figure 3.
MKK3-dependent CHOP induction in macrophages is unique to FC loading. WT and Mkk3 − / − macrophages were left untreated, FC loaded using ac-LDL plus 58035, or treated with 2.5 μg/ml tunicamycin or 2 μM thapsigargin for 7 h. Whole cell lysates were immunoblotted for phospho-p38 (top panel), total p38 (second panel), CHOP (third panel), and actin (bottom panel).
Figure 4.
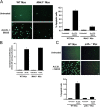
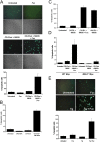
The MKK3/p38 MAPK pathway is necessary for FC-induced macrophage apoptosis. WT, Mkk3 − / − (A), and p38α-deficient (C) macrophages were left untreated or were FC loaded (ac-LDL plus 58035) for 16–18 h. The cells were stained with Alexa 488 Annexin V (green) and propidium iodide (red). Representative fluorescent images and quantitative apoptosis data from four fields of cells for each condition are shown. The data are expressed as the percent of total cells that stained with Annexin V and propidium iodide. Data are expressed as mean ± SEM (n = 4). Bar, 25 μm. (B) Esterification of [14C] oleate in WT or Mkk3 − / − macrophages incubated 5 h with medium containing [14C] oleate with or without 100 μg/ml ac-LDL. Shown is the mean ± SEM (n = 3) of cholesteryl [14C] oleate cpm per μg of total protein.
Figure 5.
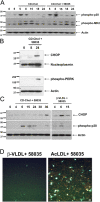
FC loading using CD-cholesterol or β-VLDL induces p38 MAPK phosphorylation and UPR induction. (A) Macrophages were left untreated for 5 h or were incubated with CD-cholesterol (CD-Chol) ± 58035 for the indicated number of hours. Whole cell lysate was immunoblotted for phospho-p38, phospho-MK2, and actin. (B) Macrophages were left untreated or incubated with CD-cholesterol plus 58035 for 8 and 24 h. Nuclear extracts were isolated as described in “Materials and methods” and were subjected to immunoblot analysis for CHOP and nucleoplasmin (as a loading control). Cytosolic extracts were immunoblotted for phospho-Thr980 PERK and actin. (C) Macrophages were incubated with CD-cholesterol or 50 μg/ml β-VLDL plus 58035 for the indicated number of hours. Whole cell lysates were subjected to immunoblot analysis for CHOP, phospho-p38, and actin. (D) Macrophages were incubated with 50 μg/ml β-VLDL or 100 μg/ml ac-LDL plus 58035 for 24 h and then stained with Alexa 488 Annexin V (green) and propidium iodide (red). Bar, 25 μm.
Figure 6.
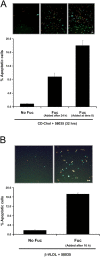
SRA ligands induce apoptosis in macrophages that are FC loaded by non-SRA sources of cholesterol, or treated with low-dose thapsigargin. (A) Macrophages were left untreated or incubated with 25 μg/ml fucoidan alone (Fuc), CD-cholesterol plus 58035 alone (CD-Chol), or CD-cholesterol/58035 plus fucoidan for 24 h. The cells were stained with Alexa 488 Annexin V (green) and propidium iodide (red). Representative fluorescent and bright field images are shown. Quantitative apoptosis data for each condition are shown as described in the legend for Fig. 4. Data are expressed as mean ± SEM (n = 8). Bar, 25 μm. (B) The experiment was conducted as in (A) except the macrophages were incubated for 24 h with 100 μg/ml of CML-BSA, CD-cholesterol/58035, or CD-cholesterol/58035 plus CML-BSA. Bar, 25 μm. As a control, FC-loaded macrophages also were treated with nonmodified BSA, which did not induce apoptosis (not depicted). (C) Quantitative apoptosis data for macrophages left untreated or incubated for 24 h with 50 μg/ml β-VLDL plus 58035, β-VLDL plus 58035 plus fucoidan (25 μg/ml), or 100 μg/ml ac-LDL plus 58035. Data are expressed as mean ± SEM (n = 8). (D) Quantitative apoptosis data for WT or Mkk3 − / − macrophages left untreated or incubated for 24 h with CD-cholesterol/58035 or CD-cholesterol/58035 plus fucoidan. Cells were stained with Alexa 488 Annexin V and propidium iodide. Representative quantitative apoptosis data are from four fields of cells under each condition. Data are expressed as mean ± SEM (n = 4). (E) Quantitative apoptosis data for macrophages left untreated or incubated for 24 h with fucoidan (25 μg/ml), 0.5 μM thapsigargin (Tg), or fucoidan plus thapsigargin. Data are expressed as mean ± SEM (n = 8). Representative fluorescent and bright field images are shown. Bar, 25 μm.
Figure 7.
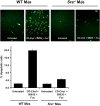
Induction of apoptosis by fucoidan plus CD-cholesterol/58035 in macrophages requires SRA expression. WT and Sra − / − macrophages were left untreated or were incubated with CD-cholesterol (CD-Chol)/58035 plus fucoidan (Fuc) for 18 h. The cells were stained with Alexa 488 Annexin V (green) and propidium iodide (red). Representative fluorescent images are shown. Quantitative apoptosis data for each condition are shown as described in the legend for Fig. 6. Data are expressed as mean ± SEM (n = 4). Bar, 25 μm.
Figure 8.
SRA engagement triggers apoptosis in macrophages that have been FC loaded before exposure to SRA ligand. (A) Macrophages were incubated with CD-cholesterol (CD-Chol)/58035 for 32 h. Some of the cells received no fucoidan (Fuc), some received fucoidan (25 μg/ml) at the beginning of the incubation period, and some received fucoidan after 24 h of incubation. (B) Macrophages were incubated with 10 μg/ml β-VLDL plus 58035 for 24 h. Some of the cells received no fucoidan and some received fucoidan after 16 h of incubation. For both sets of experiments, the cells were stained with Alexa 488 Annexin V (green) and propidium iodide (red). Representative fluorescent images are shown. Quantitative apoptosis data for each condition are shown as described in the legend for Fig. 4. Data are expressed as mean ± SEM (n = 4). Bar, 25 μm.
Figure 9.
JNK2 is necessary for apoptosis, but activation is not dependent on SRA engagement. (A) Macrophages were preincubated for 30 min with 10 μM SP600125 or with vehicle and then incubated for 18 h in medium alone or medium containing ac-LDL plus 58035 ± SP600125. The cells were stained with Alexa 488 Annexin V (green) and propidium iodide (red). Representative fluorescent images and quantitative apoptosis data from four fields of cells for each condition are shown. The data are expressed as the percent of total cells that stained with Annexin V and propidium iodide. Data are expressed as mean ± SEM (n = 4). Bar, 25 μm. (B) Macrophages were preincubated for 30 min with 15 μM SP600125 (SP) or with vehicle and then incubated for 18 h in medium containing 25 μg/ml fucoidan alone (Fuc), 0.5 μM thapsigargin (Tg) alone, or both compounds. Quantitative apoptosis data for each condition are shown as described in (A). Data are expressed as mean ± SEM (n = 4). (C) WT or Jnk2 −/− macrophages were incubated for 18 h in medium alone or medium containing ac-LDL plus 58035, 0.5 μM thapsigargin, or 0.5 μM thapsigargin plus 25 μg/ml fucoidan. Quantitative apoptosis data for each condition are shown as described in (A). Data are expressed as mean ± SEM (n = 4). (D) Macrophages were incubated with 0.5 μM thapsigargin or 0.5 μM thapsigargin plus 25 μg/ml fucoidan for 0, 1, 2, and 3 h. Whole cell lysates were prepared as described in “Materials and methods,” and were immunoblotted for activated phospho-Thr 183/Tyr185 JNK (phospho-JNK, top panel) and total JNK (bottom panel). (E) Macrophages were preincubated for 30 min with medium alone or medium containing the anti-SRA antibody 2f8 or isotype control IgG2b (30 μg/ml). The cells were incubated for 3 h in medium alone or medium containing 0.5 μM thapsigargin plus 25 μg/ml fucoidan. Whole cell lysates were prepared as described in “Materials and methods,” and were immunoblotted for activated phospho-Thr 183/Tyr185 JNK (top panel), and total JNK (bottom panel). (F) WT and Jnk2 − / − macrophages (Mφs) were FC loaded for 0 or 12, and 15 h using 100 μg/ml ac-LDL plus the ACAT inhibitor 58035. Whole cell lysates were prepared as described in “Materials and methods,” and were immunoblotted for CHOP (top panel), total JNK (middle panel), and actin (bottom panel).
Figure 10.
Model of how ER stress, MAPK pathways, and SRA engagement conspire to induce apoptosis in FC-loaded macrophages. Excess FC loading of the ER induces an ER stress response that leads to activation of p38 and JNK. Activation of p38, in turn, induces the UPR effector, CHOP. Three pathways—p38-CHOP, JNK, and engagement of the SRA—combine to induce apoptosis. Ac-LDL is able to trigger all three pathways, because it delivers cholesterol to the cells and is a ligand for the SRA.
Similar articles
- Free cholesterol-induced macrophage apoptosis is mediated by inositol-requiring enzyme 1 alpha-regulated activation of Jun N-terminal kinase.
Li F, Guo Y, Sun S, Jiang X, Tang B, Wang Q, Wang L. Li F, et al. Acta Biochim Biophys Sin (Shanghai). 2008 Mar;40(3):226-34. doi: 10.1111/j.1745-7270.2008.00396.x. Acta Biochim Biophys Sin (Shanghai). 2008. PMID: 18330477 - Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages.
Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Seimon TA, et al. Proc Natl Acad Sci U S A. 2006 Dec 26;103(52):19794-9. doi: 10.1073/pnas.0609671104. Epub 2006 Dec 13. Proc Natl Acad Sci U S A. 2006. PMID: 17167049 Free PMC article. - The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques.
Tsukano H, Gotoh T, Endo M, Miyata K, Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K, Mizuta H, Oike Y. Tsukano H, et al. Arterioscler Thromb Vasc Biol. 2010 Oct;30(10):1925-32. doi: 10.1161/ATVBAHA.110.206094. Epub 2010 Jul 22. Arterioscler Thromb Vasc Biol. 2010. PMID: 20651282 - Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice.
Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Matsuzawa A, et al. Antioxid Redox Signal. 2002 Jun;4(3):415-25. doi: 10.1089/15230860260196218. Antioxid Redox Signal. 2002. PMID: 12215209 Review. - Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis.
Linton MF, Babaev VR, Huang J, Linton EF, Tao H, Yancey PG. Linton MF, et al. Circ J. 2016 Oct 25;80(11):2259-2268. doi: 10.1253/circj.CJ-16-0924. Epub 2016 Oct 8. Circ J. 2016. PMID: 27725526 Free PMC article. Review.
Cited by
- Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development.
Quillard T, Libby P. Quillard T, et al. Circ Res. 2012 Jul 6;111(2):231-44. doi: 10.1161/CIRCRESAHA.112.268144. Circ Res. 2012. PMID: 22773426 Free PMC article. Review. - New roles of HDL in inflammation and hematopoiesis.
Zhu X, Parks JS. Zhu X, et al. Annu Rev Nutr. 2012 Aug 21;32:161-82. doi: 10.1146/annurev-nutr-071811-150709. Epub 2012 Apr 23. Annu Rev Nutr. 2012. PMID: 22540255 Free PMC article. Review. - Soat1 mediates the mouse strain effects on cholesterol loading-induced endoplasmic reticulum stress and CHOP expression in macrophages.
Luo M, Opoku E, Traughber CA, Hai Q, Robinet P, Berisha S, Smith JD. Luo M, et al. Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Jan;1866(1):158825. doi: 10.1016/j.bbalip.2020.158825. Epub 2020 Oct 6. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 33031913 Free PMC article. - An update on the lipid nephrotoxicity hypothesis.
Ruan XZ, Varghese Z, Moorhead JF. Ruan XZ, et al. Nat Rev Nephrol. 2009 Dec;5(12):713-21. doi: 10.1038/nrneph.2009.184. Epub 2009 Oct 27. Nat Rev Nephrol. 2009. PMID: 19859071 Review. - Multifaceted Nature of Lipid Droplets in Viral Interactions and Pathogenesis.
Herrera-Moro Huitron L, De Jesús-González LA, Martínez-Castillo M, Ulloa-Aguilar JM, Cabello-Gutierrez C, Helguera-Repetto C, Garcia-Cordero J, León Juárez M. Herrera-Moro Huitron L, et al. Microorganisms. 2023 Jul 21;11(7):1851. doi: 10.3390/microorganisms11071851. Microorganisms. 2023. PMID: 37513023 Free PMC article. Review.
References
- Austin, R.C., S. Lentz, and G. Werstuck. 2004. Role of hyperhomocysteinemail in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 11:S56–64. - PubMed
- Ball, R.Y., E.C. Stowers, J.H. Burton, N.R.B. Cary, J.N. Skepper, and M.J. Mitchinson. 1995. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 114:45–54. - PubMed
- Baynes, J.W., and S.R. Thorpe. 2000. Glycoxidation and lipoxidation in atherogenesis. Free Radic. Biol. Med. 28:1708–1716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK07715/DK/NIDDK NIH HHS/United States
- HL75662/HL/NHLBI NIH HHS/United States
- R01 HL057560/HL/NHLBI NIH HHS/United States
- HL57560/HL/NHLBI NIH HHS/United States
- R01 HL075662/HL/NHLBI NIH HHS/United States
- HL062311/HL/NHLBI NIH HHS/United States
- R01 HL062311/HL/NHLBI NIH HHS/United States
- T32 DK007715/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous