Dissecting the role of Rho-mediated signaling in contractile ring formation - PubMed (original) (raw)
Dissecting the role of Rho-mediated signaling in contractile ring formation
Keiju Kamijo et al. Mol Biol Cell. 2006 Jan.
Abstract
In anaphase, microtubules provide a specification signal for positioning of the contractile ring. However, the nature of the signal remains unknown. The small GTPase Rho is a potent regulator of cytokinesis, but the involvement of Rho in contractile ring formation is disputed. Here, we show that Rho serves as a microtubule-dependent signal that specifies the position of the contractile ring. We found that Rho translocates to the equatorial region before furrow ingression. The Rho-specific inhibitor C3 exoenzyme and small interfering RNA to the Rho GDP/GTP exchange factor ECT2 prevent this translocation and disrupt contractile ring formation, indicating that active Rho is required for contractile ring formation. ECT2 forms a complex with the GTPase-activating protein MgcRacGAP and the kinesinlike protein MKLP1 at the central spindle, and the localization of ECT2 at the central spindle depends on MgcRacGAP and MKLP1. In addition, we show that the bundled microtubules direct Rho-mediated signaling molecules to the furrowing site and regulate furrow formation. Our study provides strong evidence for the requirement of Rho-mediated signaling in contractile ring formation.
Figures
Figure 1.
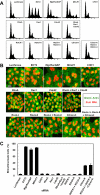
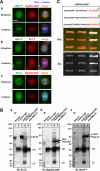
Immunoblots showing siRNA-mediated depletion of the proteins involved in Rho-mediated signaling. HeLa cells were treated with the indicated siRNAs for 72 h. GAPDH, glyceraldehyde-3-phosphate dehydrogenase, for a loading control.
Figure 2.
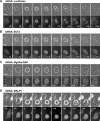
Effects of RNAi on cytokinesis. HeLa cells were treated with the indicated siRNAs for 72 h. (A) Flow cytometry profiles; horizontal axis, DNA content; vertical axis, cell counts. Positions corresponding to 2N, 4N, 8N, and 16N are indicated. (B) Morphology of siRNA-treated cells stained with 4,6-diamidino-2-phenylindole for DNA (red) and phalloidin for F-actin (green). Bar, 25 μm. (C) Frequencies of bi/multinucleate cells. In each of three independent experiments, 250 cells were examined. Values are shown as mean (%) ± SD.
Figure 3.
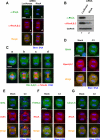
Translocation of active Rho to the equatorial region is required for contractile ring formation. (A and B) RhoA RNAi. Cells were treated with siRNA to Luciferase or RhoA for 48 h. (A) Cells stained with RhoA-specific and pan-RhoA, B, C antibodies. Bar, 10 μm. (B) Immunoblots, using RhoA-specific and pan-RhoA, B, and C antibodies. (C) Localization of Rho during mitosis. HeLa cells were fixed with 10% TCA and stained with pan-RhoA, B, and C antibody. a, metaphase; b, anaphase A; c, anaphase B; and d, telophase. Bar, 10 μm. Note that Rho translocated to the equatorial region before furrow ingression (b). (D) C3 delocalizes Rho from the equatorial region. Cells were stained with anti-RhoA and anti-RhoA, B, and C antibodies. Bar, 10 μm. (E–G) Active Rho is required for contractile ring formation. Bars, 10 μm. HeLa cells were loaded with PBS (Mock) or C3. Cells were stained with anti-myosin heavy chain (MHC) antibody and phalloidin (Actin) (E); anti-phosphorylated myosin regulatory light chain (P-MRLC) and anti-RhoA antibodies (F); anti-Citron-kinase (Citron-K) and anti-RhoA antibodies (G). In mock cells, myosin heavy chain and F-actin accumulated at the equatorial region (E; Mock); phosphorylated MRLC and Citron-kinase colocalized with RhoA at the equatorial region (F and G; Mock). In C3-loaded cells, acto-myosin contractile ring formation was disrupted (E; C3); accumulation of phosphorylated MRLC and Citron-kinase at the equatorial region (F and G; C3) were inhibited.
Figure 4.
C3 exoenzyme inhibits contractile ring formation. (A and B) HeLa cells that stably express MRLC-GFP were loaded with PBS (Mock) or C3 by the scrape-loading method. Selected images of time-lapse recording of mock cells (A; Movie S1) and C3-loaded cells (B; Movie S2). Time in minutes from the onset of anaphase (time point 0) is indicated. Bars, 10 μm. C3 inhibited accumulation of MRLC to the equatorial region (B).
Figure 5.
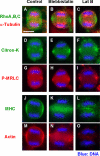
Translocation of Rho to the equatorial region is independent of actin and myosin. HeLa cells were treated with DMSO (Control), blebbistatin, or latrunculin B (Lat B). Cells were fixed and stained with anti-Rho A, B, and C, anti-α-tubulin, anti-Citron-kinase (Citron-K), anti-phosphorylated MRLC (P-MRLC), anti-myosin heavy chain (MHC) antibodies, and phalloidin (Actin).
Figure 6.
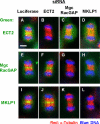
ECT2, MgcRacGAP, and MKLP1-dependent translocation of Rho to the equatorial region. HeLa cells were treated with siRNA to luciferase, ECT2, MgcRacGAP, or MKLP1 for 36 h. The cells were fixed with 10% TCA. Bars, 10 μm. (A) Cells stained with RhoA-specific and pan-RhoA, B, and C antibodies. ECT2, MgcRac-GAP, or MKLP1 RNAi diminished the level of staining for both RhoA and RhoA, B, and C. (B and C) Abnormal localization of Rho in ECT2, MgcRacGAP, or MKLP1 RNAi cells. HeLa cells were stained with anti-RhoA antibody and the localization of RhoA was examined. (B) Examples of normal and abnormal localization of RhoA. Shown are MKLP1 RNAi cells. Localization of RhoA was classified as follows: a, Normal; b– d, Aberrant; and e, Delocalized. (C) Scoring of RhoA localization in anaphase cells. (D) Cells stained with phosphorylated MRLC (P-MRLC) and Citron-kinase (Citron-K). ECT2, MgcRacGAP, or MKLP1 RNAi inhibited accumulation of Citron-kinase and phosphorylated MRLC at the equatorial region.
Figure 7.
Formation of the contractile ring depends on ECT2, MgcRacGAP, and MKLP1. (A–D) Distribution of MRLC during cell division. Selected images from time-lapse recording. HeLa cells that stably express MRLC-GFP were treated with siRNA to Luciferase (A; Movie S3), ECT2 (B; Movie S4), MgcRacGAP (C; Movie S5), and MKLP1 (D; Movie S6). Bars, 10 μm. Time in minutes from the onset of anaphase (time point 0) is indicated. ECT2, MgcRacGAP, or MKLP1 RNAi inhibited the accumulation of MRLC at the equatorial region (B–D).
Figure 8.
ECT2 forms complex with MgcRacGAP and MKLP1 at the central spindle. (A) Subcellular localization of ECT2, MgcRacGAP, and MKLP1 in metaphase and anaphase. HeLa cells were stained with indicated antibodies and anti-α-tubulin. Bar, 10 μm. In metaphase, MKLP1, MgcRacGAP, and ECT2 were found throughout in the cytoplasm. MKLP1 and MgcRacGAP were also found associated with the spindle. In anaphase, ECT2, MgcRacGAP, and MKLP1 were colocalized at the interdigitating portion of the central spindle. (B) Coimmunoprecipitation of ECT2 with MgcRacGAP and MKLP1. An extract from HeLa cells synchronized in anaphase to cytokinesis was immunoprecipitated with control IgG or antibodies to ECT2, MgcRacGAP, and MKLP1. Immunoprecipitates were separated by SDS-PAGE, transferred to polyvinylidene diflouride membrane, and probed with indicated antibodies. (C) ECT2 interacts with MgcRacGAP but not MKLP1. Interactions were tested by a yeast two-hybrid system with full-length ECT2, MgcRacGAP, and MKLP1. Plasmids (pGBDU/pGAD) were transformed into PJ69-4A cells. After the transformants were selected, the cells were grown on nonselective medium (YPD) or selective medium (–His) at 25°C for 3 d. Asterisks indicate significant interactions. GAP:MgcRacGAP.
Figure 9.
Localization of ECT2 at the central spindle depends on MgcRacGAP and MKLP1. Localization of ECT2, MgcRacGAP, and MKLP1 in cells depleted of these protein by RNAi. Bar, 10 μm. In control cells, ECT2 (A), MgcRacGAP (E), and MKLP1 (I) were localized at the interdigitating portion of the central spindle. MgcRacGAP or MKLP1 RNAi delocalized ECT2 from the central spindle (C and D) but ECT2 RNAi did not affect the localization of MgcRacGAP (F) and MKLP1 (J). The localization of MKLP1 and MgcRacGAP at the central spindle requires MgcRacGAP and MKLP1, respectively (H and K).
Figure 10.
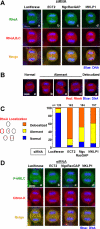
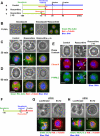
Bundled microtubules direct the Rho-signaling molecules to the furrowing site. Note that a, b, and c in C–E correspond to the experimental diagram shown in A. (A) Schematic diagram showing roscovitine treatment. Cells were treated with nocodazole for 3 h, and then nocodazole was washed out. Cells cultured without a or with roscovitine b. (c) Cells pretreated with roscovitine 15 min before nocodazole washout and then cultured with roscovitine [roscovitine (–15)]. (B) Localization of Rho (green) and microtubules (red) in cells treated without (Nocodazole) or with (Nocodazole + Roscovitine) roscovitine in the presence of nocodazole (corresponding to time point 0 min in A, a and c, respectively). In the presence of nocodazole, accumulation of Rho was inhibited. (C) Localization of Rho (green) and microtubules (red) in roscovitine-treated cells 30 min after washout of nocodazole. a, control cell at prometaphase. b, roscovitine-treated cell. Rho accumulated at ectopic furrows (arrows). Microtubules were bundled adjacent to the ectopic furrows. c, pretreatment with roscovitine suppresses furrow formation. Microtubules were not bundled and Rho did not accumulate. D, localization of Rho (green) and microtubules (red) in cells 90 min after washout of nocodazole. a, control cell in anaphase. Rho accumulated at the equatorial region. b, roscovitine-treated cells terminated contraction. Bundling of microtubules and accumulation of Rho disappeared. c, roscovitine-pretreated cells spread out again. (E) Localization of Citron-kinase (Citron-K; red) and phosphorylated MRLC (P-MRLC; green). a, 90 min; b and c, 30 min after nocodazole washout, respectively. In roscovitine-treated cells, Citron-kinase and phosphorylated MRLC accumulated at the ectopic furrows (b; arrows). Roscovitine pretreatment prevented formation of ectopic furrows and accumulation of Citron-kinase and phosphorylated MRLC (c). (F–H) RhoGEF ECT2 regulates translocation of Rho to the furrows. (F) Schematic diagram showing roscovitine treatment in luciferase or ECT2 RNAi cells. Cells were treated with siRNA to luciferase or ECT2 for 24 h and synchronized with nocodazole for 3 h. Then, nocodazole was washed out and cultured in the presence of roscovitine. (G) ECT2 staining in roscovitine-treated luciferase (a) or ECT2 (b) RNAi cells. (H) Localization of Rho in roscovitine-treated luciferase (a) or ECT2 (b) RNAi cells. In ECT2 RNAi cells, microtubules were bundled but Rho did not accumulate.
Figure 11.
Rho-mediated signaling serves as a microtubule-dependent signal for furrow positioning.
Similar articles
- MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis.
Zhao WM, Fang G. Zhao WM, et al. Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13158-63. doi: 10.1073/pnas.0504145102. Epub 2005 Aug 29. Proc Natl Acad Sci U S A. 2005. PMID: 16129829 Free PMC article. - Stabilization of anaphase midzone microtubules is regulated by Rho during cytokinesis in human fibrosarcoma cells.
Kanada M, Nagasaki A, Uyeda TQ. Kanada M, et al. Exp Cell Res. 2009 Oct 1;315(16):2705-14. doi: 10.1016/j.yexcr.2009.06.027. Epub 2009 Jul 1. Exp Cell Res. 2009. PMID: 19576212 - An ECT2-centralspindlin complex regulates the localization and function of RhoA.
Yüce O, Piekny A, Glotzer M. Yüce O, et al. J Cell Biol. 2005 Aug 15;170(4):571-82. doi: 10.1083/jcb.200501097. J Cell Biol. 2005. PMID: 16103226 Free PMC article. - Centralspindlin in Rappaport's cleavage signaling.
Mishima M. Mishima M. Semin Cell Dev Biol. 2016 May;53:45-56. doi: 10.1016/j.semcdb.2016.03.006. Epub 2016 Mar 7. Semin Cell Dev Biol. 2016. PMID: 26964770 Review. - Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy.
D'Avino PP, Savoian MS, Glover DM. D'Avino PP, et al. J Cell Sci. 2005 Apr 15;118(Pt 8):1549-58. doi: 10.1242/jcs.02335. J Cell Sci. 2005. PMID: 15811947 Review.
Cited by
- What Drosophila spermatocytes tell us about the mechanisms underlying cytokinesis.
Giansanti MG, Fuller MT. Giansanti MG, et al. Cytoskeleton (Hoboken). 2012 Nov;69(11):869-81. doi: 10.1002/cm.21063. Epub 2012 Sep 21. Cytoskeleton (Hoboken). 2012. PMID: 22927345 Free PMC article. Review. - Insights on the Role of PGRMC1 in Mitotic and Meiotic Cell Division.
Lodde V, Garcia Barros R, Terzaghi L, Franciosi F, Luciano AM. Lodde V, et al. Cancers (Basel). 2022 Nov 23;14(23):5755. doi: 10.3390/cancers14235755. Cancers (Basel). 2022. PMID: 36497237 Free PMC article. Review. - Polo-like kinase controls vertebrate spindle elongation and cytokinesis.
Brennan IM, Peters U, Kapoor TM, Straight AF. Brennan IM, et al. PLoS One. 2007 May 2;2(5):e409. doi: 10.1371/journal.pone.0000409. PLoS One. 2007. PMID: 17476331 Free PMC article. - Rho-guanine nucleotide exchange factors during development: Force is nothing without control.
Mulinari S, Häcker U. Mulinari S, et al. Small GTPases. 2010 Jul;1(1):28-43. doi: 10.4161/sgtp.1.1.12672. Small GTPases. 2010. PMID: 21686118 Free PMC article. - Small GTPase RhoA regulates cytoskeleton dynamics during porcine oocyte maturation and early embryo development.
Zhang Y, Duan X, Cao R, Liu HL, Cui XS, Kim NH, Rui R, Sun SC. Zhang Y, et al. Cell Cycle. 2014;13(21):3390-403. doi: 10.4161/15384101.2014.952967. Cell Cycle. 2014. PMID: 25485583 Free PMC article.
References
- Canman, J. C., Cameron, L. A., Maddox, P. S., Straight, A., Tirnauer, J. S., Mitchison, T. J., Fang, G., Kapoor, T. M., and Salmon, E. D. (2003). Determining the position of the cell division plane. Nature 424, 1074 –1078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous