Integrin alphaDbeta2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties - PubMed (original) (raw)
Integrin alphaDbeta2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties
Valentin P Yakubenko et al. Blood. 2006.
Abstract
Integrin alphaDbeta2, the most recently discovered member of the beta2 subfamily of integrin adhesion receptors, is up-regulated on macrophage foam cells. Although other members of the subfamily have been subjects of extensive research, the recognition specificity and the molecular basis for alphaDbeta2 ligand binding remain unknown. Based on the high extent of structural homology between alphaDbeta2 and the major myeloid-cell-specific integrin alphaMbeta2 (Mac-1), noted for its capacity to bind multiple ligands, we considered that the 2 integrins have similar recognition specificity. In this study, using recombinant and natural alphaDbeta2-expressing cells, we demonstrate that alphaDbeta2 supports adhesion and migration to many extracellular matrix proteins in a fashion similar to alphaMbeta2. Consistent with these data, the recombinant alphaDI-domain of the receptor bound selected ligands. The binding was activation-dependent because the alphaDI-domain with its C-terminal alpha7 helix truncated, but not the form with the C-terminal part extended, bound ligands. When the alphaDI-domain segment Lys244-Lys260 (highly homologous to its alphaMI-domain counterpart Lys245-Arg261 responsible for alphaMbeta2 multiligand-binding properties) was inserted into the mono-specific alphaLI-domain, the chimeric protein bound many ligands with affinities similar to those of wild-type alphaDI-domain. These results establish integrin alphaDbeta2 as a multiligand receptor and indicate that the mechanism whereby alphaDbeta2 exhibits broad ligand specificity resembles that used by alphaMbeta2, the most promiscuous member of the integrin family.
Figures
Figure 1.
Amino acid alignment of the αMI-domain sequence Lys245-Arg261 with other integrin α subunit I-domains. The αMI-domain sequence was aligned with human αX, αD, αL, α1, α2, α10, α11, and αE using the National Center for Biotechnology Informatics (NCBI) database. Numbers on the right indicate the homology between αM (assigned a value of 100%) and other α subunits expressed as the percentage of identical residues (shown in bold). Dots above the αDI-domain sequence indicate residues homologous between αD and αM. Residues identified as critical for ligand binding in the αMI-domain, are underlined.
Figure 2.
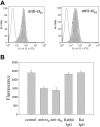
Analysis of integrin expression and heterodimer formation in HEK 293 cells transfected with wild-type αDβ2. (A) The binding of anti-αD-specific polyclonal antibody and anti-β2-specific mAb IB4 to the αDβ2-expressing cells was analyzed by flow cytometry. Results are presented as histograms with the logarithm of fluorescence intensity on the abscissa and the cell number on the ordinate. Control cells incubated with Alexa 488-conjugated secondary antibody are shown in the top panel. (B) Immunoprecipitation of biotin-labeled αDβ2. Cells (5 × 105) were labeled with biotin, lysed, and immunoprecipitated with 10 μg anti-β2 mAb IB4. The immunoprecipitates were analyzed by Western blotting using streptavidin conjugated to horseradish peroxidase. The integrin subunits were detected using an enhanced chemiluminescent substrate and exposed to Kodak BioMax film.
Figure 3.
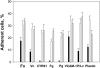
Adhesion of αDβ2- and αMβ2-expressing HEK 293 cells to different ligands. HEK 293 cells expressing wild-type αDβ2 (□) and αMβ2 ( ), and mock-transfected cells (▪), were labeled with calcein and their adhesion to different ligands was tested. Aliquots (50 μL) of 5 × 105/mL cells in DMEM/F-12 were added to the wells coated with increasing concentrations of different ligands. After incubation for 30 minutes at 37°C, the nonadherent cells were removed by 2 washes with PBS and fluorescence was measured. The number of adherent cells was calculated by using the fluorescence of aliquots with a known number of labeled cells. Maximal adhesion to each substrate is shown as was determined from the dose-dependent curves of adhesion. Maximal adhesion to fibronectin (Fn), fibrinogen (Fg), plasminogen (Pg), Cyr61, vitronectin (Vn), P2-C, and VCAM-1 was observed at coating concentrations of 10 μg/mL, 2 μg/mL, 10 μg/mL, 2.5 μg/mL, 5 μg/mL, 50 μg/mL, and 5 μg/mL, respectively. Data are expressed as a percentage of added cells and are the mean ± SE of 3 to 6 individual experiments.
), and mock-transfected cells (▪), were labeled with calcein and their adhesion to different ligands was tested. Aliquots (50 μL) of 5 × 105/mL cells in DMEM/F-12 were added to the wells coated with increasing concentrations of different ligands. After incubation for 30 minutes at 37°C, the nonadherent cells were removed by 2 washes with PBS and fluorescence was measured. The number of adherent cells was calculated by using the fluorescence of aliquots with a known number of labeled cells. Maximal adhesion to each substrate is shown as was determined from the dose-dependent curves of adhesion. Maximal adhesion to fibronectin (Fn), fibrinogen (Fg), plasminogen (Pg), Cyr61, vitronectin (Vn), P2-C, and VCAM-1 was observed at coating concentrations of 10 μg/mL, 2 μg/mL, 10 μg/mL, 2.5 μg/mL, 5 μg/mL, 50 μg/mL, and 5 μg/mL, respectively. Data are expressed as a percentage of added cells and are the mean ± SE of 3 to 6 individual experiments.
Figure 4.
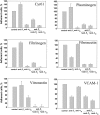
Effect of function blocking antibodies on adhesion of the αDβ2-expressing HEK 293 cells. Calcein-labeled αDβ2-expressing HEK 293 cells were preincubated for 20 minutes at 22°C with anti-β1 polyclonal antibody 1950 (1:500 dilution), 20 μg/mL anti-αD polyclonal antibody, or with combinations of anti-β1 with either anti-αD or anti-β2 mAb IB4 (20 μg/mL). Cells were added to microtiter wells coated with various ligands at concentrations that produce maximal adhesion, and cell adhesion was determined as described in Figure 3. Data are expressed as a percentage of control (adhesion in the absence of antibodies) and are the mean ± SE of 3 individual experiments performed in triplicate in each experiment.
Figure 5.
Expression of αDβ2 and αMβ2 on the surface of IC-21 macrophage cell line and their role in macrophage migration. (A) The level of αDβ2 and αMβ2 expression was assessed by flow cytometry with mAb 1/70, which recognizes mouse αMβ2, and polyclonal anti-αD antibody, which recognizes both human and mouse αD integrin subunits. Control cells are shown as open histograms. (B) IC-21 cells were analyzed for their ability to migrate to 10 μg/mL vitronectin either in the absence or in the presence of blocking polyclonal anti-αD and anti-αM mAb M1/70. Cells were preincubated with 2.5 μg/mL of each antibody for 20 minutes before their addition to the upper chamber of Transwell plates. In control samples, cells were pretreated with 2.5 μg/mL of each rabbit or rat IgG. Cells were allowed to migrate toward vitronectin for 18 hours at 37°C and the extent of cell migration was assessed as described in “Materials and methods.”
Figure 6.
SDS-PAGE of generated I-domains. The I-domains were isolated from soluble fractions of E coli lysates and purified using affinity chromatography, and their purity was assessed by SDS-PAGE on a 12.5% gel under reducing conditions followed by staining with Coomassie blue.
Figure 7.
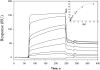
Analyses of the αDI-domain binding to vitronectin by SPR. Representative profiles of the SPR responses for αDI-domain binding (concentrations ranging from 0.05 to 3 μM) to vitronectin coupled to the CM5 chip. The “active” αDI-domain (Pro128-Lys314) in HBS-P buffer supplemented with 1 mM MgCl2 was used. RU indicates response units. The inset shows a dose-dependent saturable binding of the αDI-domain to vitronectin.
Similar articles
- The Role of Integrins αMβ2 (Mac-1, CD11b/CD18) and αDβ2 (CD11d/CD18) in Macrophage Fusion.
Podolnikova NP, Kushchayeva YS, Wu Y, Faust J, Ugarova TP. Podolnikova NP, et al. Am J Pathol. 2016 Aug;186(8):2105-2116. doi: 10.1016/j.ajpath.2016.04.001. Epub 2016 Jun 14. Am J Pathol. 2016. PMID: 27315778 Free PMC article. - Oxidative modifications of extracellular matrix promote the second wave of inflammation via β2 integrins.
Yakubenko VP, Cui K, Ardell CL, Brown KE, West XZ, Gao D, Stefl S, Salomon RG, Podrez EA, Byzova TV. Yakubenko VP, et al. Blood. 2018 Jul 5;132(1):78-88. doi: 10.1182/blood-2017-10-810176. Epub 2018 May 3. Blood. 2018. PMID: 29724896 Free PMC article. - Multiple binding sites in fibrinogen for integrin alphaMbeta2 (Mac-1).
Lishko VK, Podolnikova NP, Yakubenko VP, Yakovlev S, Medved L, Yadav SP, Ugarova TP. Lishko VK, et al. J Biol Chem. 2004 Oct 22;279(43):44897-906. doi: 10.1074/jbc.M408012200. Epub 2004 Aug 10. J Biol Chem. 2004. PMID: 15304494 - β2 Integrin CD11d/CD18: From Expression to an Emerging Role in Staged Leukocyte Migration.
Blythe EN, Weaver LC, Brown A, Dekaban GA. Blythe EN, et al. Front Immunol. 2021 Nov 8;12:775447. doi: 10.3389/fimmu.2021.775447. eCollection 2021. Front Immunol. 2021. PMID: 34858434 Free PMC article. Review. - Integrins and their ligands.
Sonnenberg A. Sonnenberg A. Curr Top Microbiol Immunol. 1993;184:7-35. doi: 10.1007/978-3-642-78253-4_2. Curr Top Microbiol Immunol. 1993. PMID: 8313723 Review.
Cited by
- Toll-like receptors and integrins crosstalk.
Alhamdan F, Bayarsaikhan G, Yuki K. Alhamdan F, et al. Front Immunol. 2024 Jun 10;15:1403764. doi: 10.3389/fimmu.2024.1403764. eCollection 2024. Front Immunol. 2024. PMID: 38915411 Free PMC article. Review. - Regulating Blood Clot Fibrin Films to Manipulate Biomaterial-Mediated Foreign Body Responses.
Zou Y, Shan Z, Han Z, Yang J, Lin Y, Gong Z, Xie L, Xu J, Xie R, Chen Z, Chen Z. Zou Y, et al. Research (Wash D C). 2023 Sep 15;6:0225. doi: 10.34133/research.0225. eCollection 2023. Research (Wash D C). 2023. PMID: 37719049 Free PMC article. - Targeting integrin pathways: mechanisms and advances in therapy.
Pang X, He X, Qiu Z, Zhang H, Xie R, Liu Z, Gu Y, Zhao N, Xiang Q, Cui Y. Pang X, et al. Signal Transduct Target Ther. 2023 Jan 2;8(1):1. doi: 10.1038/s41392-022-01259-6. Signal Transduct Target Ther. 2023. PMID: 36588107 Free PMC article. Review. - Modification of Extracellular Matrix by the Product of DHA Oxidation Switches Macrophage Adhesion Patterns and Promotes Retention of Macrophages During Chronic Inflammation.
Casteel JL, Keever KR, Ardell CL, Williams DL, Gao D, Podrez EA, Byzova TV, Yakubenko VP. Casteel JL, et al. Front Immunol. 2022 May 26;13:867082. doi: 10.3389/fimmu.2022.867082. eCollection 2022. Front Immunol. 2022. PMID: 35720381 Free PMC article. - The Role of CCN1 in Esophageal Adenocarcinoma: What We Have Learned From the Lab.
Chang Z, Dang T, Meng X, Chai J. Chang Z, et al. Cancer Control. 2022 Jan-Dec;29:10732748221074734. doi: 10.1177/10732748221074734. Cancer Control. 2022. PMID: 35291889 Free PMC article. Review.
References
- Van der Vieren M, Le Trong H, St.John T, Staunton DE, Gallatin WM. A novel leukointegrin, αdβ2, binds preferentially to ICAM-3. Immunity. 1995;3: 683-690. - PubMed
- Noti JD. Expression of the myeloid-specific leukocyte integrin gene CD11d during macrophage foam cell differentiation and exposure to lipoproteins. Int J Mol Med. 2002;10: 721-727. - PubMed
- Borregaard N, Miller LJ, Springer TA. Chemoattractant-regulated fusion of a novel, mobilizable intracellular compartment with the plasma membrane in human neutrophils. Science. 1987;237: 1204-1206. - PubMed
- Gray JL, Shankar R. Down regulation of CD11b and CD18 expression in atherosclerotic lesion-derived macrophages. Am Surg. 1995;61: 674-679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials