Pharmacological induction of heat-shock proteins alleviates polyglutamine-mediated motor neuron disease - PubMed (original) (raw)
Pharmacological induction of heat-shock proteins alleviates polyglutamine-mediated motor neuron disease
Masahisa Katsuno et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Spinal and bulbar muscular atrophy (SBMA) is an adult-onset motor neuron disease caused by the expansion of a trinucleotide CAG repeat encoding the polyglutamine tract in the first exon of the androgen receptor gene (AR). The pathogenic, polyglutamine-expanded AR protein accumulates in the cell nucleus in a ligand-dependent manner and inhibits transcription by interfering with transcriptional factors and coactivators. Heat-shock proteins (HSPs) are stress-induced chaperones that facilitate the refolding and, thus, the degradation of abnormal proteins. Geranylgeranylacetone (GGA), a nontoxic antiulcer drug, has been shown to potently induce HSP expression in various tissues, including the central nervous system. In a cell model of SBMA, GGA increased the levels of Hsp70, Hsp90, and Hsp105 and inhibited cell death and the accumulation of pathogenic AR. Oral administration of GGA also up-regulated the expression of HSPs in the central nervous system of SBMA-transgenic mice and suppressed nuclear accumulation of the pathogenic AR protein, resulting in amelioration of polyglutamine-dependent neuromuscular phenotypes. These observations suggest that, although a high dose appears to be needed for clinical effects, oral GGA administration is a safe and promising therapeutic candidate for polyglutamine-mediated neurodegenerative diseases, including SBMA.
Figures
Fig. 1.
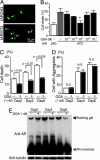
Effects of GGA on polyglutamine toxicity in cultured cell. (A) Punctuated aggregates visualized with GFP (arrowhead) are formed in SHSY-5Y cells infected with an adenovirus vector containing truncated AR with 97 CAGs (tAR97Q-GFP) but not in those bearing tAR24Q. (B) Frequency of cell death 6 days after infection as detected by propidium iodine staining (*, P < 0.05 compared with untreated tAR97Q cells). (C) Suppression of cell death by GGA. (D) Frequency of cells bearing aggregates. (E) Anti-AR analysis of Western blots of extracts from cells infected with tAR97Q. Error bars indicate SD.
Fig. 2.
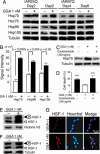
GGA-mediated HSP induction in cultured cell. (A) Anti-HSP analysis of Western blots from cells infected with tAR97Q and treated with GGA. (B) Quantification of the levels of HSPs from tAR97Q-infected cells after 2 days of GGA treatment. (C) Anti-Hsp70 analysis of Western blots from tAR97Q cells treated with or without cycloheximide. (D) Frequency of cell death 2 days after infection as detected by propidium iodine staining (**, P < 0.05 compared with tAR97Q cells treated with GGA but not with cycloheximide). (E and F) Anti-HSF-1 analysis of Western blots of the cellular nuclear fraction (E) and that of total cell lysate (F). Upper bands correspond to the hyperphosphorylated, active form of HSF-1. (G) Immunocytochemistry for HSF-1. Error bars indicate SD.
Fig. 3.
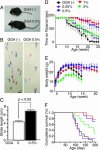
Effect of GGA on neurological phenotypes of AR-97Q mice. (A) Muscle atrophy of 13-week AR-97Q mice. (B) Footprints of 13-week AR-97Q mice. Front paws are shown in red, and hind paws are shown in blue. (C) Stride distance of 13-week AR-97Q mice (n = 3 for each group). (_D_-F) Rotarod task (D), body weight (E), and cumulative survival (F) of male AR-97Q mice treated with GGA (n = 12 for each group) and untreated counterparts (n = 15). Rotarod performance significantly improved after GGA at doses of 0.5% and 1.0% (P < 0.0001 at both doses compared with nontreated mice at 20 weeks), and body weight increased significantly at a dose of 0.5% (P < 0.005 at 0.5% and P < 0.05 at 1.0%, at 14 weeks). Error bars indicate SD.
Fig. 4.
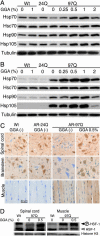
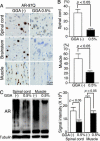
GGA-mediated HSP induction in AR-97Q mice. (A) Western blotting for various HSPs in the spinal cord of 14-week, wild-type (Wt), AR-24Q and AR-97Q mice. (B) Western blotting for various HSPs in skeletal muscle of 14-week wild-type, AR-24Q, and AR-97Q mice. (C) Immunohistochemistry for Hsp70 in 14-week wild-type, AR-24Q, and AR-97Q mice. (D) Western blotting of nuclear fraction from spinal cord and that from muscle using anti-HSF-1 antibody. Upper bands correspond to the hyperphosphorylated active form of HSF-1.
Fig. 5.
Effect of GGA on accumulation of abnormal AR. (A) Immunohistochemistry of 14-week wild-type, AR-24Q, and AR-97Q mice using 1C2 antibody. (B) Quantification of 1C2-positive cells in spinal cord and muscle of AR-97Q mice treated with or without GGA. (C) Western blotting for AR of 14-week AR-97Q mice and quantification of the high-molecular-weight, abnormal AR complex indicated by a smear from the top of the gel. Error bars indicate SD.
Similar articles
- Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein.
Adachi H, Katsuno M, Minamiyama M, Sang C, Pagoulatos G, Angelidis C, Kusakabe M, Yoshiki A, Kobayashi Y, Doyu M, Sobue G. Adachi H, et al. J Neurosci. 2003 Mar 15;23(6):2203-11. doi: 10.1523/JNEUROSCI.23-06-02203.2003. J Neurosci. 2003. PMID: 12657679 Free PMC article. - [Triplet repeat disease, with particular emphasis of spinal and bulbar muscular atrophy (SBMA)].
Sobue G. Sobue G. Rinsho Shinkeigaku. 2000 Dec;40(12):1193-5. Rinsho Shinkeigaku. 2000. PMID: 11464455 Japanese. - Pathogenesis and therapy of spinal and bulbar muscular atrophy (SBMA).
Katsuno M, Tanaka F, Adachi H, Banno H, Suzuki K, Watanabe H, Sobue G. Katsuno M, et al. Prog Neurobiol. 2012 Dec;99(3):246-56. doi: 10.1016/j.pneurobio.2012.05.007. Epub 2012 May 15. Prog Neurobiol. 2012. PMID: 22609045 Review. - Spinal and bulbar muscular atrophy: ligand-dependent pathogenesis and therapeutic perspectives.
Katsuno M, Adachi H, Tanaka F, Sobue G. Katsuno M, et al. J Mol Med (Berl). 2004 May;82(5):298-307. doi: 10.1007/s00109-004-0530-7. Epub 2004 Feb 27. J Mol Med (Berl). 2004. PMID: 15133611 Review. - CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model.
Adachi H, Waza M, Tokui K, Katsuno M, Minamiyama M, Tanaka F, Doyu M, Sobue G. Adachi H, et al. J Neurosci. 2007 May 9;27(19):5115-26. doi: 10.1523/JNEUROSCI.1242-07.2007. J Neurosci. 2007. PMID: 17494697 Free PMC article.
Cited by
- Heat shock factor-1 influences pathological lesion distribution of polyglutamine-induced neurodegeneration.
Kondo N, Katsuno M, Adachi H, Minamiyama M, Doi H, Matsumoto S, Miyazaki Y, Iida M, Tohnai G, Nakatsuji H, Ishigaki S, Fujioka Y, Watanabe H, Tanaka F, Nakai A, Sobue G. Kondo N, et al. Nat Commun. 2013;4:1405. doi: 10.1038/ncomms2417. Nat Commun. 2013. PMID: 23360996 - Aggregation formation in the polyglutamine diseases: protection at a cost?
Todd TW, Lim J. Todd TW, et al. Mol Cells. 2013 Sep;36(3):185-94. doi: 10.1007/s10059-013-0167-x. Epub 2013 Jun 19. Mol Cells. 2013. PMID: 23794019 Free PMC article. Review. - Molecular Mechanism for Various Pharmacological Activities of NSAIDS.
Mizushima T. Mizushima T. Pharmaceuticals (Basel). 2010 May 25;3(5):1614-1636. doi: 10.3390/ph3051614. Pharmaceuticals (Basel). 2010. PMID: 27713320 Free PMC article. Review. - Reversible disruption of dynactin 1-mediated retrograde axonal transport in polyglutamine-induced motor neuron degeneration.
Katsuno M, Adachi H, Minamiyama M, Waza M, Tokui K, Banno H, Suzuki K, Onoda Y, Tanaka F, Doyu M, Sobue G. Katsuno M, et al. J Neurosci. 2006 Nov 22;26(47):12106-17. doi: 10.1523/JNEUROSCI.3032-06.2006. J Neurosci. 2006. PMID: 17122035 Free PMC article. - Chaperones and proteases: cellular fold-controlling factors of proteins in neurodegenerative diseases and aging.
Hinault MP, Ben-Zvi A, Goloubinoff P. Hinault MP, et al. J Mol Neurosci. 2006;30(3):249-65. doi: 10.1385/JMN:30:3:249. J Mol Neurosci. 2006. PMID: 17401151 Review.
References
- Zoghbi, H. Y. & Orr, H. T. (2000) Annu. Rev. Neurosci. 23, 217-247. - PubMed
- Ross, C. A. (2002) Neuron 35, 819-822. - PubMed
- Kennedy, W. R., Alter, M. & Sung, J. H. (1968) Neurology 18, 671-680. - PubMed
- Sobue, G., Hashizume, Y., Mukai, E., Hirayama, M., Mitsuma, T. & Takahashi, A. (1989) Brain 112, 209-232. - PubMed
- La Spada, A. R., Wilson, E. M., Lubahn, D. B., Harding, A. E. & Fischbeck, K. H. (1991) Nature 352, 77-79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous