Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy - PubMed (original) (raw)
Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy
Juhua Zhou et al. J Immunol. 2005.
Abstract
Recent studies have indicated that adoptive immunotherapy with autologous antitumor tumor-infiltrating lymphocytes (TILs) following nonmyeloablative chemotherapy mediates tumor regression in approximately 50% of treated patients with metastatic melanoma, and that tumor regression is correlated with the degree of persistence of adoptively transferred T cells in peripheral blood. These findings, which suggested that the proliferative potential of transferred T cells may play a role in clinical responses, led to the current studies in which telomere length as well as phenotypic markers expressed on the administered TILs were examined. TILs that were associated with objective clinical responses following adoptive transfer possessed a mean telomere length of 6.3 kb, whereas TILs that were not associated with significant clinical responses were significantly shorter, averaging 4.9 kb (p < 0.01). Furthermore, individual TIL-derived T cell clonotypes that persisted in vivo following adoptive cell transfer possessed telomeres that were longer than telomeres of T cell clonotypes that failed to persist (6.2 vs 4.5 kb, respectively; p < 0.001). Expression of the costimulatory molecule CD28 also appeared to be associated with long telomeres and T cell persistence. These results, indicating that the telomere length of transferred lymphocytes correlated with in vivo T cell persistence following adoptive transfer, and coupled with the previous observation that T cell persistence was associated with clinical responses in this adoptive immunotherapy trial, suggest that telomere length and the proliferative potential of the transferred T cells may play a significant role in mediating response to adoptive immunotherapy.
Figures
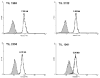
Figure 1
Representative histograms of telomere flow-FISH assays of bulk administered TILs. Administered TIL samples were thawed and used in telomere flow-FISH assays without further culture. Shaded area represents the unstained control.
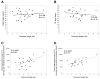
Figure 2
Telomere length of administered TIL is not correlated with age of TIL or patient age but is correlated with absolute lymphocyte count. The relationship was examined between telomere lengths of the administered TIL and patient age (A), or between the telomere lengths of the administered TILs and the TIL age (B). TIL age refers to the total number of days TIL were cultured in vitro. The association between the telomere lengths of the TIL prior to adoptive transfer and absolute lymphocyte counts in the peripheral blood obtained between 5 and 10 days following adoptive cell transfer (C) and between 19 and 37 days following adoptive cell transfer (D) was evaluated. All TIL samples were included if their corresponding data were available.
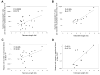
Figure 3
Correlation of telomere length of transferred lymphocytes with in vivo T cell persistence. The telomere lengths of transferred lymphocytes were compared with the total number of persistent T cells detected in patient peripheral blood samples obtained between 5 and 10 days following adoptive cell transfer (A) and between 19 and 37 days following adoptive transfer (B). In addition, the relative number of persistent T cells that were present in peripheral blood was calculated by determining the ratio of number of cells corresponding to individual persistent clonotypes that were originally present in the administered TIL to the number of those cells detected in peripheral blood at the two time points, assuming a total cell blood volume of 4 liters. A value of 1 indicates that the number of cells present in blood was equal to that present in the TIL. The relative cell numbers were calculated between 5 and 10 days (C) and between 19 and 37 days (D) following transfer. All clonotypes that were present in the administered TILs, as determined by sequencing the expressed TRBV gene products, and that were also detected in the peripheral blood at levels of 1% or greater in either sample, were included in this analysis.
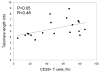
Figure 4
Relationship between telomere lengths and CD28 expression of transferred T cell clonotypes. The percentage of CD28 expression on cells corresponding to individual clonotypes was determined by co-staining with anti-TCR Vbeta antibodies. Closed squares represented persistent clonotypes and closed circles represented non-persistent clonotypes.
Similar articles
- Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression.
Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, Robbins PF. Huang J, et al. J Immunother. 2005 May-Jun;28(3):258-67. doi: 10.1097/01.cji.0000158855.92792.7a. J Immunother. 2005. PMID: 15838383 Free PMC article. - Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy.
Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Zhou J, et al. J Immunother. 2005 Jan-Feb;28(1):53-62. doi: 10.1097/00002371-200501000-00007. J Immunother. 2005. PMID: 15614045 Free PMC article. - Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length.
Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ Jr, Rosenberg SA, Hodes RJ. Shen X, et al. J Immunother. 2007 Jan;30(1):123-9. doi: 10.1097/01.cji.0000211321.07654.b8. J Immunother. 2007. PMID: 17198091 Free PMC article. - Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook.
Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, Chen JQ, Hwu P, Radvanyi LG. Wu R, et al. Cancer J. 2012 Mar-Apr;18(2):160-75. doi: 10.1097/PPO.0b013e31824d4465. Cancer J. 2012. PMID: 22453018 Free PMC article. Review. - Tumor-infiltrating lymphocyte therapy for melanoma: rationale and issues for further clinical development.
Sim GC, Chacon J, Haymaker C, Ritthipichai K, Singh M, Hwu P, Radvanyi L. Sim GC, et al. BioDrugs. 2014 Oct;28(5):421-37. doi: 10.1007/s40259-014-0097-y. BioDrugs. 2014. PMID: 24890028 Review.
Cited by
- Comprehensive snapshots of natural killer cells functions, signaling, molecular mechanisms and clinical utilization.
Chen S, Zhu H, Jounaidi Y. Chen S, et al. Signal Transduct Target Ther. 2024 Nov 8;9(1):302. doi: 10.1038/s41392-024-02005-w. Signal Transduct Target Ther. 2024. PMID: 39511139 Free PMC article. Review. - Higher TIGIT+ γδ TCM cells may predict poor prognosis in younger adult patients with non-acute promyelocytic AML.
Hou Q, Wang P, Kong X, Chen J, Yao C, Luo X, Li Y, Jin Z, Wu X. Hou Q, et al. Front Immunol. 2024 Apr 22;15:1321126. doi: 10.3389/fimmu.2024.1321126. eCollection 2024. Front Immunol. 2024. PMID: 38711501 Free PMC article. - Biomarkers for response to TIL therapy: a comprehensive review.
Albarrán Fernández V, Ballestín Martínez P, Stoltenborg Granhøj J, Borch TH, Donia M, Marie Svane I. Albarrán Fernández V, et al. J Immunother Cancer. 2024 Mar 13;12(3):e008640. doi: 10.1136/jitc-2023-008640. J Immunother Cancer. 2024. PMID: 38485186 Free PMC article. Review. - Nonactivated and IL-7 cultured CD19-specific CAR T cells are enriched in stem cell phenotypes and functionally superior.
Wang SY, Scurti GM, Dalheim AV, Quinn S, Stiff PJ, Nishimura MI. Wang SY, et al. Blood Adv. 2024 Jan 23;8(2):324-335. doi: 10.1182/bloodadvances.2023010607. Blood Adv. 2024. PMID: 37967375 Free PMC article. - TCR-engaging scaffolds selectively expand antigen-specific T-cells with a favorable phenotype for adoptive cell therapy.
Tvingsholm SA, Frej MS, Rafa VM, Hansen UK, Ormhøj M, Tyron A, Jensen AWP, Kadivar M, Bentzen AK, Munk KK, Aasbjerg GN, Ternander JSH, Heeke C, Tamhane T, Schmess C, Funt SA, Kjeldsen JW, Kverneland AH, Met Ö, Draghi A, Jakobsen SN, Donia M, Marie Svane I, Hadrup SR. Tvingsholm SA, et al. J Immunother Cancer. 2023 Aug;11(8):e006847. doi: 10.1136/jitc-2023-006847. J Immunother Cancer. 2023. PMID: 37586765 Free PMC article.
References
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. - PMC - PubMed
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials