Key stages in mammary gland development: the mammary end bud as a motile organ - PubMed (original) (raw)
Review
Key stages in mammary gland development: the mammary end bud as a motile organ
Lindsay Hinck et al. Breast Cancer Res. 2005.
Abstract
In the rodent, epithelial end buds define the tips of elongating mammary ducts. These highly motile structures undergo repeated dichotomous branching as they aggressively advance through fatty stroma and, turning to avoid other ducts, they finally cease growth leaving behind the open, tree-like framework on which secretory alveoli develop during pregnancy. This review identifies the motility of end buds as a unique developmental marker that represents the successful integration of systemic and local mammotrophic influences, and covers relevant advances in ductal growth regulation, extracellular matrix (ECM) remodeling, and cell adhesion in the inner end bud. An unexpected growth-promoting synergy between insulin-like growth factor-1 and progesterone, in which ducts elongate without forming new end buds, is described as well as evidence strongly supporting self-inhibition of ductal elongation by end-bud-secreted transforming growth factor-beta acting on stromal targets. The influence of the matrix metalloproteinase ECM-remodeling enzymes, notably matrix metalloproteinase-2, on end bud growth is discussed in the broader context of enzymes that regulate the polysaccharide-rich glycosaminoglycan elements of the ECM. Finally, a critical, motility-enabling role for the cellular architecture of the end bud is identified and the contribution of cadherins, the netrin/neogenin system, and ErbB2 to the structure and motility of end buds is discussed.
Figures
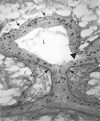
Figure 1
Photomicrographs illustrating motility and histoarchitecture of end buds. (a) Natural and experimentally induced motility 'behavior' of end buds in the mammary ductal system of a 5-week-old nulliparous mouse. The 'open' ductal architecture of the mammary tree leaves 80% or more of the gland epithelium-free. Large terminal end buds identify the most actively growing region of the gland (top arrows), and progressively smaller lateral end buds extend to each side of the center, indicating slowed forward growth as the end bud encounters a thinning fat pad. End buds may also reverse direction to grow back into accommodating stroma (side arrow). Bifurcating end buds (top arrows) are arrayed along the growth front. Original magnification approx. ×12. (b) Cross-section through end bud with accompanying diagram. End buds are bilayered structures; an outer layer of myoepithelial progenitor cells (cap cells) overlays a multilayered mass of luminal cells fated to form the walls of the ductal lumen (L). Stained with hematoxylin and eosin. Original magnification approx. ×300.
Figure 2
Photomicrograph of a longitudinal section through an end bud and its subtending duct. A lateral end bud stained to reveal sulfated glycosaminoglycans and mitotic cells with the cap cell layer is indicated by the dashed line. Constriction of the end bud to ductal dimensions coincides with induction of a collagenous extracellular matrix along the end bud flank. Note that this sheath is continuous with the subtending duct as well as the duct of origin (dotted lines). The basal lamina along the end bud flank (large arrows) as well as in the cleft of a bifurcation (triangle) stained deeply for sulfated glycosaminoglycans (Alcian blue stain). This contrasts with weakly stained basal lamina around the end bud tip indicative of non-sulfated hyaluronate (short arrows). Silver grains (dark spots) are from tritiated thymidine autoradiography and mark mitotic cells in the end bud and subjacent ducts. Original magnification approx. ×300.
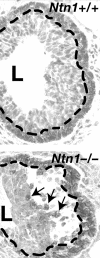
Figure 3
Loss of Ntn1 disorganizes end buds. The Ntn1+/+ end bud (top) displays normal CDH3 staining of the cap cell layer at the tip of the end bud (delineated by the dashed line). In contrast the _Ntn1_-/- end bud (bottom) displays a loss of adhesion between the cap and luminal cell layers, with a large space forming under the cap cell layer (delineated by the dashed line). This space fills with dissociated cap cells (arrows show three examples) that either die by apoptosis or migrate inappropriately into the body of the end bud. Original magnification approx. ×300.
Similar articles
- Regulation of mammary morphogenesis: evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-beta 1.
Silberstein GB, Flanders KC, Roberts AB, Daniel CW. Silberstein GB, et al. Dev Biol. 1992 Aug;152(2):354-62. doi: 10.1016/0012-1606(92)90142-4. Dev Biol. 1992. PMID: 1644225 - Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse.
Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, Streuli CH. Klinowska TC, et al. Dev Biol. 1999 Nov 1;215(1):13-32. doi: 10.1006/dbio.1999.9435. Dev Biol. 1999. PMID: 10525347 - Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis.
Williams JM, Daniel CW. Williams JM, et al. Dev Biol. 1983 Jun;97(2):274-90. doi: 10.1016/0012-1606(83)90086-6. Dev Biol. 1983. PMID: 6852366 - TGF beta regulation of cell proliferation.
Moses HL, Arteaga CL, Alexandrow MG, Dagnino L, Kawabata M, Pierce DF Jr, Serra R. Moses HL, et al. Princess Takamatsu Symp. 1994;24:250-63. Princess Takamatsu Symp. 1994. PMID: 8983080 Review. - Tumour-stromal interactions. Role of the stroma in mammary development.
Silberstein GB. Silberstein GB. Breast Cancer Res. 2001;3(4):218-23. doi: 10.1186/bcr299. Epub 2001 Mar 22. Breast Cancer Res. 2001. PMID: 11434872 Free PMC article. Review.
Cited by
- Dynamics of branched tissue assembly.
Manivannan S, Nelson CM. Manivannan S, et al. Stem Cell Res Ther. 2012 Oct 31;3(5):42. doi: 10.1186/scrt133. Stem Cell Res Ther. 2012. PMID: 23114096 Free PMC article. Review. - Morphogenesis of the developing mammary gland: stage-dependent impact of adipocytes.
Landskroner-Eiger S, Park J, Israel D, Pollard JW, Scherer PE. Landskroner-Eiger S, et al. Dev Biol. 2010 Aug 15;344(2):968-78. doi: 10.1016/j.ydbio.2010.06.019. Epub 2010 Jun 19. Dev Biol. 2010. PMID: 20599899 Free PMC article. - Loss of cyclin D1 in concert with deregulated estrogen receptor alpha expression induces DNA damage response activation and interrupts mammary gland morphogenesis.
Frech MS, Torre KM, Robinson GW, Furth PA. Frech MS, et al. Oncogene. 2008 May 15;27(22):3186-93. doi: 10.1038/sj.onc.1210974. Epub 2007 Dec 10. Oncogene. 2008. PMID: 18071314 Free PMC article. - Intraductal Delivery and X-ray Visualization of Ethanol-Based Ablative Solution for Prevention and Local Treatment of Breast Cancer in Mouse Models.
Kenyon E, Zaluzec EK, Powell K, Volk M, Chakravarty S, Hix J, Arora R, Westerhuis JJ, Kiupel M, Shapiro EM, Sempere LF. Kenyon E, et al. J Vis Exp. 2022 Apr 1;(182):10.3791/63457. doi: 10.3791/63457. J Vis Exp. 2022. PMID: 35435915 Free PMC article.
References
- Nandi S. Endocrine control of mammary gland development and function in the C3H/He Crgl mouse. J Natl Cancer Inst. 1958;21:1039–1063. - PubMed
- Lyons WR. Hormonal synergism in mammary growth. Proc R Soc Lond B. 1958;149:303–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous