Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells - PubMed (original) (raw)
Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells
Hiroyuki Tada et al. Infect Immun. 2005 Dec.
Abstract
A synthetic Nod2 agonist, muramyldipeptide (MDP), and two Nod1 agonists, FK565 and FK156, mimic the bacterial peptidoglycan moiety and are powerful adjuvants that induce cell-mediated immunity, especially delayed-type hypersensitivity. In this study, we used human dendritic cell (DC) cultures to examine possible T helper type 1 (Th1) responses induced by MDP and FK565/156 in combination with various synthetic Toll-like receptor (TLR) agonists, including synthetic lipid A (TLR4 agonist), the synthetic triacyl lipopeptide Pam3CSSNA (TLR2 agonist), poly(I:C) (TLR3 agonist), and CpG DNA (TLR9 agonist). Immature DCs derived from human monocytes expressed mRNAs for Nod1, Nod2, TLR2, TLR3, TLR4, and TLR9. The stimulation of DCs with MDP and FK565 in combination with lipid A, poly(I:C), and CpG DNA, but not with Pam3CSSNA, synergistically induced interleukin-12 (IL-12) p70 and gamma interferon (IFN-gamma), but not IL-18, in culture supernatants and induced IL-15 on the cell surface. In correlation with the cytokine induction, an upregulation of the mRNA expression of these cytokine genes was observed. Notably, IL-12 p35 mRNA expression increased >1,000-fold upon stimulation with lipid A plus either MDP or FK565 compared with stimulation with each stimulant alone. In contrast, for the expression of CD83 and costimulatory molecules such as CD40, CD80, and CD86, no synergistic effects were observed upon stimulation with Nod plus TLR agonists. The culture supernatants of DCs stimulated with lipid A plus either MDP or FK565 activated human T cells to produce high levels of IFN-gamma, and the activity was attributable to DC-derived IL-12. These findings suggest that Nod1 and Nod2 agonists in combination with TLR3, TLR4, and TLR9 agonists synergistically induce IL-12 and IFN-gamma production in DCs to induce Th1-lineage immune responses.
Figures
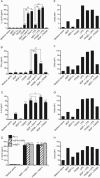
FIG. 1.
Expression of Nods and TLRs in DCs derived from human monocytes. (A) Preparation of DCs from monocytes. Human monocytes were cultured in the presence of IL-4 (100 ng/ml) and GM-CSF (100 ng/ml) for 6 days. Cells were collected and stained with anti-HLA-DR (FITC) and anti-CD1a (PE) by flow cytometry to confirm the purity of DCs. (B) Phenotypes of DCs. The DCs were collected and stained with anti-CD1a, -HLA-DR, -CD83, -CD14, -CD40, -CD80, and -CD86 (solid lines) or matched isotype MAbs (dotted lines) and analyzed by flow cytometry. The results in panels A to C are representative of three independent experiments. The percentage of gated cells (A) and the mean fluorescence intensity (B) are indicated in the corner. (C) mRNA expression for Nods and TLRs in DCs. Total RNA was extracted from the cells and analyzed for Nod1, Nod2, TLR2, TLR3, TLR4, TLR9, and β-actin by RT-PCR.
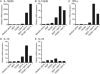
FIG. 2.
Synergistic effect of MDP or FK565 with lipid A or LPS on induction of IL-12 p70 and IFN-γ, but not IL-8, and upregulation of CD83, CD80, CD86, and CD40 expression on DCs. DCs were stimulated for 72 h at 37°C with MDP (10 μg/ml), FK565 (10 μg/ml), MDP-LL (10 μg/ml), lipid A (10 ng/ml), and LPS (10 ng/ml). DCs were pretreated with an anti-IFN-γ MAb (10 μg/ml) or a matched isotype MAb (10 μg/ml) for 30 min at 37°C and then were stimulated with MDP, FK565, and LPS for 24 h (H). The amounts of IL-12 p70 (A and D), IFN-γ (B), and IL-8 (C) in the culture supernatants were analyzed by ELISA. Cells were stained with anti-CD83 (E), -CD80 (F), -CD86 (G), and -CD40 (H) or with matched isotype MAbs and analyzed by flow cytometry. The results indicate the mean fluorescence intensities (MFI). Data from ELISAs are expressed as mean values ± SD, and statistically significant differences are shown. ** and *, P < 0.01 and P < 0.05, respectively, versus medium-alone control; ## and #, P < 0.01 and P < 0.05, respectively, versus lipid A or LPS alone. The results are representative of three independent experiments.
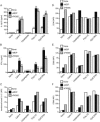
FIG. 3.
Synergistic effect of MDP or FK565 with lipid A on induction of IL-12 p35, IL-12 p40, IFN-γ, and IL-15 mRNA expression in DCs. DCs were stimulated for 6 h (or 24 h for IL-15 mRNA) at 37°C with MDP (10 μg/ml), FK565 (10 μg/ml), and lipid A (10 ng/ml). Total RNA was extracted from the cells, and reverse transcription of the RNA samples to cDNAs was performed. Real-time PCR amplification was carried out using a LightCycler-FastStart DNA Master SYBR green I kit (Roche Diagnostics). The relative induction of IL-12 p35 (A), IL-12 p40 (B), IFN-γ (C), IL-15 (D), and IL-18 (E) mRNA expression was determined after normalization using β-actin. The results are representative of three independent experiments.
FIG. 4.
Synergistic effect of Nod agonists with TLR agonists on induction of IL-12 p70 and IFN-γ production and on upregulation of membrane-bound IL-15, CD40, CD80, and CD86 expression on DCs. DCs were stimulated for 72 h at 37°C with MDP (10 μg/ml), FK565 (10 μg/ml), lipid A (10 ng/ml), Pam3CSSNA (1 μM, 1.27 μg/ml), poly(I:C) (10 μg/ml), or CpG DNA (2 μM). The amounts of IL-12 p70 (A) and IFN-γ (B) in the culture supernatants were analyzed by ELISA. Data are expressed as mean values ± SD, and significant differences are shown. ** and *, P < 0.01 and P < 0.05, respectively, versus medium alone; ## and #, P < 0.01 and P < 0.05, respectively, versus the respective TLR agonist alone. Cells were stained with anti-human IL-15 (C), -CD40 (D), -CD80 (E), and -CD86 (F) or with matched isotype MAbs and analyzed by flow cytometry. The results indicate the mean fluorescence intensities (MFI). The results are representative of three independent experiments.
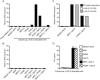
FIG. 5.
Combinatory stimulation of DCs by MDP or FK565 with lipid A induces IL-12, which in turn induces IFN-γ mRNA expression in T cells. (A) DCs were stimulated for 72 h at 37°C with MDP (10 μg/ml), FK565 (10 μg/ml), MDP-LL (10 μg/ml), lipid A (10 ng/ml), or various combinations of the compounds. After cultivation, the culture supernatants of DCs were collected. Human peripheral blood T cells were stimulated with the culture supernatants. (B) As a control experiment, T cells were also stimulated with the respective stimulants for 24 h at 37°C. (C) DC culture supernatants were pretreated with an anti-IL-12 MAb (10 μg/ml) or anti-IL-18 MAb (1 μg/ml) for 30 min at 37°C. The total RNA was then extracted from the T cells (A to C), and real-time PCR analyses were carried out. The relative induction of IFN-γ mRNA expression was determined after normalization using β-actin. (D) The amount of IFN-γ in the culture supernatants was analyzed by ELISA. The results are representative of three different experiments. Data are expressed as mean values ± SD, and statistically significant differences are shown. **, P < 0.01 versus medium alone.
Similar articles
- Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture.
Uehara A, Yang S, Fujimoto Y, Fukase K, Kusumoto S, Shibata K, Sugawara S, Takada H. Uehara A, et al. Cell Microbiol. 2005 Jan;7(1):53-61. doi: 10.1111/j.1462-5822.2004.00433.x. Cell Microbiol. 2005. PMID: 15617523 - Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists.
Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, Cavaillon JM, Philpott DJ, Adib-Conquy M. Fritz JH, et al. Eur J Immunol. 2005 Aug;35(8):2459-70. doi: 10.1002/eji.200526286. Eur J Immunol. 2005. PMID: 16021602 - Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, NOD1 and NOD2 in human oral epithelial cells.
Uehara A, Sugawara Y, Kurata S, Fujimoto Y, Fukase K, Kusumoto S, Satta Y, Sasano T, Sugawara S, Takada H. Uehara A, et al. Cell Microbiol. 2005 May;7(5):675-86. doi: 10.1111/j.1462-5822.2004.00500.x. Cell Microbiol. 2005. PMID: 15839897 - Enhancement of TLR-mediated innate immune responses by peptidoglycans through NOD signaling.
Takada H, Uehara A. Takada H, et al. Curr Pharm Des. 2006;12(32):4163-72. doi: 10.2174/138161206778743510. Curr Pharm Des. 2006. PMID: 17100619 Review. - Enhancement of endotoxin activity by muramyldipeptide.
Takada H, Yokoyama S, Yang S. Takada H, et al. J Endotoxin Res. 2002;8(5):337-42. doi: 10.1179/096805102125000669. J Endotoxin Res. 2002. PMID: 12537692 Review.
Cited by
- Nucleotide-binding oligomerization domain 2 signaling promotes hyperresponsive macrophages and colitis in IL-10-deficient mice.
Jamontt J, Petit S, Clark N, Parkinson SJ, Smith P. Jamontt J, et al. J Immunol. 2013 Mar 15;190(6):2948-58. doi: 10.4049/jimmunol.1201332. Epub 2013 Feb 8. J Immunol. 2013. PMID: 23396949 Free PMC article. - NLRP12 in innate immunity and inflammation.
Tuladhar S, Kanneganti TD. Tuladhar S, et al. Mol Aspects Med. 2020 Dec;76:100887. doi: 10.1016/j.mam.2020.100887. Epub 2020 Aug 22. Mol Aspects Med. 2020. PMID: 32838963 Free PMC article. Review. - Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases.
Correa RG, Milutinovic S, Reed JC. Correa RG, et al. Biosci Rep. 2012 Dec;32(6):597-608. doi: 10.1042/BSR20120055. Biosci Rep. 2012. PMID: 22908883 Free PMC article. Review. - Evaluation of an intranasal virosomal vaccine against respiratory syncytial virus in mice: effect of TLR2 and NOD2 ligands on induction of systemic and mucosal immune responses.
Shafique M, Meijerhof T, Wilschut J, de Haan A. Shafique M, et al. PLoS One. 2013 Apr 8;8(4):e61287. doi: 10.1371/journal.pone.0061287. Print 2013. PLoS One. 2013. PMID: 23593453 Free PMC article. - Combined Tbet and IL12 gene therapy elicits and recruits superior antitumor immunity in vivo.
Qu Y, Chen L, Lowe DB, Storkus WJ, Taylor JL. Qu Y, et al. Mol Ther. 2012 Mar;20(3):644-51. doi: 10.1038/mt.2011.283. Epub 2012 Jan 3. Mol Ther. 2012. PMID: 22215017 Free PMC article.
References
- Adam, A. 1985. Synthetic adjuvants. John Wiley & Sons, New York, N.Y.
- Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. - PubMed
- Askew, D., R. S. Chu, A. M. Krieg, and C. V. Harding. 2000. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J. Immunol. 165:6889-6895. - PubMed
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
- Bessler, W. G., R. B. Johnson, K. Wiesmüller, and G. Jung. 1982. B-lymphocyte mitogenicity in vitro of a synthetic lipopeptide fragment derived from bacterial lipoprotein. Hoppe-Seyler's Z. Physiol. Chem. 363:767-770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous