Isolation and characterization of human mammary stem cells - PubMed (original) (raw)
Review
Isolation and characterization of human mammary stem cells
R B Clarke. Cell Prolif. 2005 Dec.
Abstract
Since stem cells are present throughout the lifetime of an organism, it is thought that they may accumulate mutations, eventually leading to cancer. In the breast, tumours are predominantly oestrogen and progesterone receptor-positive (ERalpha/PR+). We therefore studied the biology of ERalpha/PR-positive cells and their relationship to stem cells in normal human mammary epithelium. We demonstrated that ERalpha/PR-positive cells co-express the putative stem cell markers p21(CIP1/WAF1), cytokeratin (CK) 19 and Musashi-1 when examined using dual label immunofluorescence on tissue sections. Next, we isolated a Hoechst dye-effluxing 'side population' (SP) from the epithelium using flow cytometry and demonstrated them to be undifferentiated cells by lack of expression of myoepithelial and luminal cell-specific antigens such as CALLA and MUC1. Epithelial SP cells were shown to be enriched for the putative stem cell markers p21(CIP1/WAF1), Musashi-1 and ERalpha/PR-positive cells. Lastly, SP cells, compared to non-SP, were highly enriched for the capacity to produce colonies containing multiple lineages in 3D basement membrane (Matrigel) culture. We conclude that breast stem cells include two populations: a primitive ERalpha/PR-negative stem cell necessary for development and a shorter term ERalpha/PR-positive stem cell necessary for adult tissue homeostasis during menstrual cycling. We speculate these two basic stem cell types may therefore be the cells of origin for ERalpha-positive and -negative breast tumours.
Figures
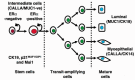
Figure 1
A model of the cellular hierarchies that may exist in the epithelium of the human breast lobule. Cells in an intermediate position include Hoechst dye‐effluxing SP cells and some of these express steroid receptors, p21CIP1, Msi1 and CK19. Msi1 and Delta/Notch signalling may regulate self‐renewal of the stem cell and production of the transit‐amplifying population through a process of asymmetric cell division. After a small number of cell divisions, transit‐amplifying cells exit from the cell cycle and differentiate into myo‐ or luminal epithelial cell lineages characterized by the markers CALLA and CK14 (myoepithelial) or MUC1 and CK18 (luminal).
Similar articles
- Regulation of human breast epithelial stem cells.
Clarke RB, Anderson E, Howell A, Potten CS. Clarke RB, et al. Cell Prolif. 2003 Oct;36 Suppl 1(Suppl 1):45-58. doi: 10.1046/j.1365-2184.36.s.1.5.x. Cell Prolif. 2003. PMID: 14521515 Free PMC article. Review. - A putative human breast stem cell population is enriched for steroid receptor-positive cells.
Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. Clarke RB, et al. Dev Biol. 2005 Jan 15;277(2):443-56. doi: 10.1016/j.ydbio.2004.07.044. Dev Biol. 2005. PMID: 15617686 - Growth and differentiation of progenitor/stem cells derived from the human mammary gland.
Clayton H, Titley I, Vivanco Md. Clayton H, et al. Exp Cell Res. 2004 Jul 15;297(2):444-60. doi: 10.1016/j.yexcr.2004.03.029. Exp Cell Res. 2004. PMID: 15212947 - Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast.
Stingl J, Eaves CJ, Kuusk U, Emerman JT. Stingl J, et al. Differentiation. 1998 Aug;63(4):201-13. doi: 10.1111/j.1432-0436.1998.00201.x. Differentiation. 1998. PMID: 9745711 - The mammary gland "side population": a putative stem/progenitor cell marker?
Smalley MJ, Clarke RB. Smalley MJ, et al. J Mammary Gland Biol Neoplasia. 2005 Jan;10(1):37-47. doi: 10.1007/s10911-005-2539-0. J Mammary Gland Biol Neoplasia. 2005. PMID: 15886885 Review.
Cited by
- Identification of a subset of breast carcinomas characterized by expression of cytokeratin 15: relationship between CK15+ progenitor/amplified cells and pre-malignant lesions and invasive disease.
Celis JE, Gromova I, Cabezón T, Gromov P, Shen T, Timmermans-Wielenga V, Rank F, Moreira JM. Celis JE, et al. Mol Oncol. 2007 Dec;1(3):321-49. doi: 10.1016/j.molonc.2007.09.004. Epub 2007 Sep 25. Mol Oncol. 2007. PMID: 19383306 Free PMC article. - Evaluation of the current knowledge limitations in breast cancer research: a gap analysis.
Thompson A, Brennan K, Cox A, Gee J, Harcourt D, Harris A, Harvie M, Holen I, Howell A, Nicholson R, Steel M, Streuli C. Thompson A, et al. Breast Cancer Res. 2008;10(2):R26. doi: 10.1186/bcr1983. Epub 2008 Mar 27. Breast Cancer Res. 2008. PMID: 18371194 Free PMC article. Review. - Lifespan Extension and Sustained Expression of Stem Cell Phenotype of Human Breast Epithelial Stem Cells in a Medium with Antioxidants.
Wang KH, Kao AP, Chang CC, Lin TC, Kuo TC. Wang KH, et al. Stem Cells Int. 2016;2016:4591310. doi: 10.1155/2016/4591310. Epub 2016 Oct 11. Stem Cells Int. 2016. PMID: 27807451 Free PMC article. - Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation.
Gu B, Sun P, Yuan Y, Moraes RC, Li A, Teng A, Agrawal A, Rhéaume C, Bilanchone V, Veltmaat JM, Takemaru K, Millar S, Lee EY, Lewis MT, Li B, Dai X. Gu B, et al. J Cell Biol. 2009 Jun 1;185(5):811-26. doi: 10.1083/jcb.200810133. J Cell Biol. 2009. PMID: 19487454 Free PMC article.
References
- Behbod F, Rosen JM (2005) Will cancer stem cells provide new therapeutic targets? Carcinogenesis 26, 703–711. - PubMed
- Cairns J (1975) Mutation selection and the natural history of cancer. Nature 255, 197–200. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous