NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels - PubMed (original) (raw)
NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels
Xuming Zhang et al. EMBO J. 2005.
Abstract
Nociceptors, or pain-sensitive receptors, are unique among sensory receptors in that their sensitivity is increased by noxious stimulation. This process, called sensitization or hyperalgesia, is mediated by a variety of proinflammatory factors, including bradykinin, ATP and NGF, which cause sensitization to noxious heat stimuli by enhancing the membrane current carried by the heat- and capsaicin-gated ion channel, TRPV1. Several different mechanisms for sensitization of TRPV1 have been proposed. Here we show that NGF, acting on the TrkA receptor, activates a signalling pathway in which PI3 kinase plays a crucial early role, with Src kinase as the downstream element which binds to and phosphorylates TRPV1. Phosphorylation of TRPV1 at a single tyrosine residue, Y200, followed by insertion of TRPV1 channels into the surface membrane, explains most of the rapid sensitizing actions of NGF.
Figures
Figure 1
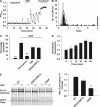
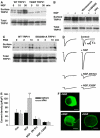
NGF enhances TRPV1 function and total phosphorylation. (A) Calcium-dependent fluorescence, F, relative to maximal fluorescence, F_max, as a function of time from a single HEK293 cell stably transfected with TrkA and transiently transfected with hTRPV1. Pulses of capsaicin (100 nM, applied as shown at top) elicited submaximal increases in [Ca]i. Exposure to NGF (100 ng/ml, see top) enhanced the capsaicin-induced calcium increase. Arrows show responses used for calculation of sensitization ratio (see B). (B) Frequency distributions of ratios between Δ_F for arrowed capsaicin applications in (A), with NGF (black bars) and without NGF (open bars). Ratios greater than 8 collected at right. Continuous curve shows Gaussian function fitted to data without NGF. Arrow shows the upper 95% confidence limit of Gaussian. Cells with ratios above this value were categorized as sensitized. The mean Δ_F_ ratio enhancement following NGF application, averaged over all capsaicin-responsive cells, was 3.30±0.38 (mean±s.e.m., _n_=125). (C) Percentage of cells sensitized without (first bar) and with NGF (100 ng/ml), and with an inhibitor of PI3 kinase (wortmannin, 100 nM) and of PKA (H89, 10 μM). The final bar shows sensitization with S502A/S801A TRPV1. Error bars show s.e.m.; significance levels (see Materials and methods) given relative to the data in NGF (second bar). (D) Time course of increase in total phosphorylation. Mean ratios (_n_=2) between TRPV1 phosphor-specific (ProQ Diamond) and total protein (SyPro Ruby) fluorescence intensities. Ratios normalized to that before exposure to NGF. (E) Comparison between phosphor-specific fluorescence increase following exposure to NGF (100 ng/ml, 40 min) in WT TRPV1, and in S502A/S801A and Y200F mutant TRPV1. Pro-Q Diamond fluorescence at the top, restained with SyPro Ruby for total protein at the bottom. Arrows show the position of the main TRPV1 band. TRPV1 appears as multiple bands because of variable glycosylation (Jahnel et al, 2001). (F) NGF-dependent phosphor-specific fluorescence increase, relative to control, in experiments similar to that shown in (E), for WT TRPV1 and TRPV1 mutants S502A/S801A and Y200F (_n_=3).
Figure 2
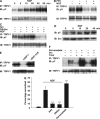
Src kinase and tyrosine phosphorylation of TRPV1. (A) Increase in tyrosine phosphorylation (pY) of TRPV1 on exposure to NGF (100 ng/ml). TRPV1 immunoprecipitated with anti-V5 antibody (IP: TRPV1) and probed for tyrosine phosphorylation with 4G-10 antibody (IB: pY). Blot then stripped and reprobed with anti-V5 to quantify total TRPV1 (IB: TRPV1). (B) Basal and NGF-induced pY are inhibited by the Src inhibitor PP2. Cells pretreated with 10 μM PP2 for 1 h before application of NGF (100 ng/ml, 30 min). Densities of bands in the top gel, relative to the first band, are 1.00, 1.32, 0.45, and 0.54. (C) Basal and NGF-induced pY or TRPV1 are enhanced by cotransfection of c-Src (lanes 3 and 4) and inhibited by cotransfection of dominant-negative Src (lanes 5 and 6). Band densities 1.00, 1.31, 1.39, 1.67, 0.64, and 0.61. (D) Src tyrosine kinase activity increases following NGF exposure. Cells transfected with TrkA and Src exposed to NGF as indicated. In vitro tyrosine kinase assay using acid-denatured enolase as substrate (see Materials and methods). Blot stripped and reprobed with anti-Src to quantify total Src (lower). (E) Src-dependent tyrosine phosphorylation of TRPV1 is reduced by SHP-1 and enhanced by dominant-negative SHP-1 C455S. Cells transfected with hTRPV1 and c-Src, and with SHP-1 constructs as indicated. (F) The tyrosine phosphatase inhibitor pervanadate (250 μM for 20 min) enhanced TRPV1 tyrosine phosphorylation (lanes 1 and 2). The effect is greater when either TrkA (lanes 3 and 4) or Src (lanes 5 and 6) is cotransfected. (G) Functional enhancement of TRPV1-dependent Ca influx by NGF is inhibited by the Src inhibitor PP2 (10 μM) or by dominant-negative Src. Pervanadate (1 mM for 4 min) enhances TRPV1 gating. See Figure 1A–C for other details. Significance levels (see Materials and methods) relative to NGF (second bar) except for pervanadate data, which are relative to the bar without NGF.
Figure 3
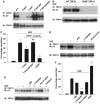
Src binds to and phosphorylates TRPV1. (A) Association between endogenous Src and TRPV1 is promoted by NGF. Src immunoprecipitated with B-12 antibody and TRPV1 association probed with anti-V5. Blot reprobed with anti-Src (middle blot). Lower blot shows total cell lysate (TCL) probed with anti-V5 for TRPV1 expression. (B) Src phosphorylates TRPV1 in vitro. See details in Materials and methods. (C) SH3 domain of Src binds to TRPV1. GST-tagged fragments of Src (as shown) used to precipitate TRPV1 from TCL. Src domains were 150–247 (SH2) and 83–144 (SH3). (D) Src binds to a proline-rich domain in the N-terminal region of TRPV1. TRPV1 phosphorylation probed with 4G-10 antibody (upper blot) following transfection of cells with DNA constructs as shown. Lower blot shows TRPV1. (E) Src binds to N- and C-terminal fragments of TRPV1. GST-tagged fragments of TRPV1 (N-TRPV1: residues 1–433; C-TRPV1: residues 681–839) used to precipitate Src from the TCL of Src-transfected HEK293 cells. Blot probed with anti-Src B-12 antibody (upper) and reprobed with anti-GST antibody (lower). Locations of Src- and GST-tagged TRPV1 fragments shown with arrows. (F) Effect of deletions from TRPV1 on basal tyrosine phosphorylation. ND: Δ1–433; CD: Δ681–839; CD 777–820: Δ777–820. Locations of TRPV1 and its mutants are shown with arrows.
Figure 4
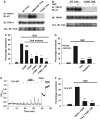
Pathways and target sites leading to TRPV1 functional enhancement. (A) Target sites on TRPV1 for Src-dependent tyrosine phosphorylation. Cells transfected with TRPV1 and c-Src (no TrkA). (B) Y200F TRPV1 mutation abolishes both basal and NGF-induced tyrosine phosphorylation. Cells transfected with TRPV1 and TrkA (no c-Src). (C) Effect of TRPV1 tyrosine residue mutations on functional enhancement of TRPV1 by NGF. See Figure 1A for experimental details. (D) Effect of Src inhibitor PP2 (10 μM) and PI3 kinase inhibitors LY294002 (25 μM) and wortmannin (100 nM) on NGF-stimulated tyrosine phosphorylation of TRPV1. (E) Effect of inhibitors as shown on tyrosine phosphrylation of TRPV1. H-89, 10 μM; LY294002, 25 μM; Ro-31-8220, 2 μM; rottlerin, 10 μM. Relative band densities 1.00, 1.27, 1.76, 0.79, 2.35, and 0.58. (F) Inhibition of functional enhancement of TRPV1 by H89, 10 μM; LY294002, 25 μM; Ro-31-8220, 500 nM. The last bar shows the effect of PMA, 1 μM. Significance levels in C and F relative to second bar (see Materials and methods).
Figure 5
TrkA residues initiating sensitization. (A) Effect of rat TrkA mutations on basal tyrosine phosphorylation of TRPV1. (B) TrkA Y760F mutation abolishes basal and NGF-stimulated TRPV1 tyrosine phosphorylation. Relative band densities 1.00, 1.32, 0.20, and 0.20. (C) Effect of TrkA tyrosine residue mutations on functional enhancement of TRPV1 by NGF. The final two bars show the effect of Y794F and Y760F TrkA mutants with S502A/S801A TRPV1 (DM). (D) Functional enhancement of TRPV1 in mouse DRG neurons by NGF inhibited by PP2 (10 μM) and rottlerin (10 μM). (E) Activation of TRPV1 by pH 5.5. Capsaicin sensitivity tested at the end. Other details as in Figure 1A. (F) Activation of TRPV1 by protons potentiated by NGF but inhibited with Y200F TRPV1 or Y760F TrkA with S502A/S801A TRPV1. Other details as in Figures 4C and 5C. Significance levels in C, D and F relative to second bar (see Materials and methods).
Figure 6
NGF enhances surface membrane expression of TRPV1. (A) Surface membrane expression of WT TRPV1 is enhanced by NGF, but Y200F TRPV1 expression is low and not affected by NGF. Surface TRPV1 isolated as in Materials and methods. Increase in WT surface membrane TRPV1 1.6±0.22-fold after 10 min (P<5%) and 2.4±0.18-fold after 30 min (P<0.1%, _n_=4). (B) Wortmannin, LY294002 and PP2 block NGF-dependent increase in surface membrane TRPV1. Relative band densities 1.00, 1.54, 1.08, 1.02, and 1.05. (C) PMA enhances surface membrane TRPV1, and enhancement is not inhibited by mutations S502A/S801A. Relative band densities 1.00, 0.77, 1.64, 1.18, 0.80, and 1.70. (D) Inward currents activated by 1-s pulses of 10 μM capsaicin in HEK293 cells cotransfected with TrkA and TRPV1. Traces shown for each condition were obtained 10 min apart with NGF (100 ng/ml) applied between pulses (apart from in first trace). Trace 3 with DN Src, trace 4 with Y200F TRPV1. (E) Collected results of patch-clamp experiments carried out as in (D). Each bar is the mean of at least seven experiments. (F) NGF promotes membrane insertion of TRPV1 in mouse DRG neurons. TRPV1 immunoreactivity imaged as described in Materials and methods. Scale bar 10 μm.
Figure 7
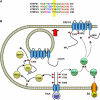
(A) Alignment of sequences adjacent to Y200 for human TRPV1–4. The bottom line shows conserved residues (*), which include the TRPV1 Y200 site (yellow bar). (B) Schematic diagram of the signaling pathways important in sensitization of TRPV1 by TrkA. The functionally most significant pathway is shown at the left (yellow, solid arrows). A smaller component of sensitization following exposure to NGF is mediated by phosphorylation of TRPV1 at residues S502 and S801, probably by the PLCγ/PKCɛ pathway (green, dashed arrows). PKCɛ is a crucial intermediate in sensitization of TRPV1 by bradykinin (pathway shown at the lower right of the diagram).
Similar articles
- Activation of CB1 inhibits NGF-induced sensitization of TRPV1 in adult mouse afferent neurons.
Wang ZY, McDowell T, Wang P, Alvarez R, Gomez T, Bjorling DE. Wang ZY, et al. Neuroscience. 2014 Sep 26;277:679-89. doi: 10.1016/j.neuroscience.2014.07.041. Epub 2014 Aug 1. Neuroscience. 2014. PMID: 25088915 Free PMC article. - Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization.
Zhuang ZY, Xu H, Clapham DE, Ji RR. Zhuang ZY, et al. J Neurosci. 2004 Sep 22;24(38):8300-9. doi: 10.1523/JNEUROSCI.2893-04.2004. J Neurosci. 2004. PMID: 15385613 Free PMC article. - Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1.
Zhu W, Oxford GS. Zhu W, et al. Mol Cell Neurosci. 2007 Apr;34(4):689-700. doi: 10.1016/j.mcn.2007.01.005. Epub 2007 Jan 24. Mol Cell Neurosci. 2007. PMID: 17324588 Free PMC article. - Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.
St Pierre M, Reeh PW, Zimmermann K. St Pierre M, et al. Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review. - Neurotrophic Factors and Nociceptor Sensitization.
Jankowski MP, Koerber HR. Jankowski MP, et al. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. PMID: 21882462 Free Books & Documents. Review.
Cited by
- Targeting TRPV1 for Cancer Pain Relief: Can It Work?
Szallasi A. Szallasi A. Cancers (Basel). 2024 Feb 2;16(3):648. doi: 10.3390/cancers16030648. Cancers (Basel). 2024. PMID: 38339399 Free PMC article. Review. - Peripheral mechanisms of arthritic pain: A proposal to leverage large animals for in vitro studies.
Chakrabarti S, Ai M, Henson FMD, Smith ESJ. Chakrabarti S, et al. Neurobiol Pain. 2020 Jul 28;8:100051. doi: 10.1016/j.ynpai.2020.100051. eCollection 2020 Aug-Dec. Neurobiol Pain. 2020. PMID: 32817908 Free PMC article. Review. - Increased nerve growth factor signaling in sensory neurons of early diabetic rats is corrected by electroacupuncture.
Nori SL, Rocco ML, Florenzano F, Ciotti MT, Aloe L, Manni L. Nori SL, et al. Evid Based Complement Alternat Med. 2013;2013:652735. doi: 10.1155/2013/652735. Epub 2013 Apr 21. Evid Based Complement Alternat Med. 2013. PMID: 23710226 Free PMC article. - Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress.
Sappington RM, Sidorova T, Ward NJ, Chakravarthy R, Ho KW, Calkins DJ. Sappington RM, et al. Channels (Austin). 2015;9(2):102-13. doi: 10.1080/19336950.2015.1009272. Channels (Austin). 2015. PMID: 25713995 Free PMC article. - Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice.
Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Amadesi S, et al. J Physiol. 2006 Sep 1;575(Pt 2):555-71. doi: 10.1113/jphysiol.2006.111534. Epub 2006 Jun 22. J Physiol. 2006. PMID: 16793902 Free PMC article.
References
- Barik S, Galinski MS (1991) ‘Megaprimer' method of PCR: increased template concentration improves yield. Biotechniques 10: 489–490 - PubMed
- Besson JM, Chaouch A (1987) Peripheral and spinal mechanisms of nociception. Physiol Rev 67: 67–155 - PubMed
- Bessou P, Perl ER (1969) Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol 32: 1025–1043 - PubMed
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous