Synthetic double-stranded RNA poly(I:C) as a potent peptide vaccine adjuvant: therapeutic activity against human cervical cancer in a rodent model - PubMed (original) (raw)
Synthetic double-stranded RNA poly(I:C) as a potent peptide vaccine adjuvant: therapeutic activity against human cervical cancer in a rodent model
Zhengrong Cui et al. Cancer Immunol Immunother. 2006 Oct.
Abstract
Due to the inherent lack of immunogenicity of peptides, it is generally recognized that the strong inflammatory signals that are required to elicit specific responses against peptide-based therapeutic tumor vaccines may not be provided by the standard/conventional vaccine adjuvants. In this study, we have demonstrated dsRNA in the form of synthetic pI:C as a potent adjuvant to enhance the specific anti-tumor immune responses against a peptide-based vaccine. When complexed with an MHC I-restricted minimal peptide epitope derived from the HPV 16 E7 protein, the resulting pI:C/E7(49-57) molecular complex induced strong E7(49-57)-specific CTL responses that caused significant regressions of model human cervical cancer tumors pre-established in mice. In addition, although the proportion of DCs in tumor-bearing mice was significantly decreased when compared to that in naïve mice, immunization with pI:C/E7(49-57 )restored the proportion of DCs in tumor-bearing mice. Double-stranded RNA may hold a great potential as an adjuvant to induce cellular immune responses for tumor immunotherapy.
Figures
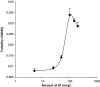
Fig. 1
Complexation of pI:C with E749–57. One hundred μl of pI:C solution (500 μg/ml) was gently mixed with an equal volume of E749–57 solution containing various amount of E749–57 peptide. After 15 min of incubation at room temperature, the turbidity of the suspension was determined (OD655). Data shown are mean ± S.D. (_n_=3)
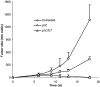
Fig. 2
Immunization of TC-1 tumor-bearing mice with pI:C/E749–57 caused extensive tumor regressions. C57BL/6 mice were s.c. injected in the flank with TC-1 cells (5×105) on day 0. On days 4 and 7, they were immunized by s.c. injection with pI:C/E749–57(_n_=10) or pI:C alone (_n_=5). Another group of mice (_n_=5) was left untreated. The dose of E749–57 was 20 μg/mouse; pI:C was dosed at 50 μg/mouse. Data shown are one representative from two independent experiments, which had similar results
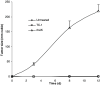
Fig. 3
The anti-tumor responses induced by pI:C/E749–57 was specific to TC-1 tumors. Mice were s.c. injected with TC-1 cells (5×105) on day 0. On days 4 and 7, they were immunized with pI:C/E749–57 (_n_=10). After the tumor regression (53 days post the initial TC-1 cell injection), mice were re-challenged with 24JK cells (5×105), TC-1 cells (5×105), or left untreated. Similar results were obtained when this experiment was repeated twice. Data shown are mean ± S.D. from one of the experiments
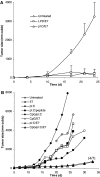
Fig. 4
a The tumor therapeutic activity induced by pI:C/E749–57 complex was comparable to that induced by E749–57 incorporated in LPD particles. Mice (_n_=7) were seeded with TC-1 tumor cells (5×105) on day 0. On day 6, they were immunized with either pI:C/E749–57,LPD/E749–57, or left untreated. On days 17, 21, and 24, two out of seven (2/7), 3/7, and 4/7 of mice in the pI:C/E749–57 immunized group became tumor-free. Data shown are mean ± S.D. b The tumor therapeutic activity induced by pI:C/E749–57complex was stronger than that induced by E749–57adjuvanted with CpG1826 oligos. Mice (_n_=5–7) were seeded with TC-1 tumor cells (5×105) on day 0. On days 4 and 7, they were immunized with either pI:C alone, pI:C/E749–57,pI:C complexed with a 9 aa non-specific peptide (pI:C/9 aa peptide), CpG1826 admixed with E749–57, CpG1826 and pI:C mixture, pI:C/E749–57 admixed with CpG1826, or left untreated. The dose for peptide, pI:C, and CpG1826 was 20, 50, and 20 μg/mouse, respectively. Mice immunized with pI:C/E749–57or pI:C/CpG/E749–57 were monitored for 35 days, at which time all of them were still alive. Mice in other groups were monitored until their death. Only mean values of the tumor size are shown to clearly illustrate the trend of tumor growth. The (4/7) indicates that 4 out of 7 mice immunized with pI:C/E749–57 or pI:C/CpG/E749–57 were tumor-free on day 35
Fig. 5
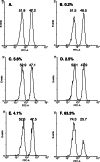
Immunization with pI:C/E749–57 induced E749–57-specific CTL responses. Mice (_n_=3) were immunized with pI:C/E749–57 (f), pI:C/9 aa peptide (e), pI:C alone (50 μg) (d), E749–57 alone (20 μg) (c), or left untreated (b) on days 0 and 7. On day 20, E749–57-specific CTL activity induced in the mice was assessed using an in vivo CTL assay. E749–57-pulsed, CFSEhighand unpulsed, CFSElow splenocytes isolated from naïve mice (10×106 each) were injected into mice via the tail vein. Mice were sacrificed 3 h later, and their splenocyte suspension was prepared and analyzed by flow cytometry. One representative from three mice, which showed similar CTL activity, is shown. The experiment was repeated twice. In flow cytometry graphs, the peak in the left represents the unpulsed CFSElow splenocytes, and that in the right represents the E749–57-pulsed, CFSEhigh splenocytes. Shown in a was the E749–57-pulsed, CFSEhigh and unpulsed CFSElow splenocyte mixture prior to injection. Numbers shown in the left-upper corner of each graph were the % of E749–57-specific CTL killing activity. Numbers shown above each peak were the relative percent of CFSElow versus the CSFEhigh cells
Fig. 6
E749–57-specific CTL responses were only detected in tumor-bearing mice treated with the pI:C/E749–57complex. Mice (_n_=3) were seeded with TC-1 cells (5×105) on day 0. On days 4 and 10, they were treated with pI:C (c), pI:C/E749–57 complex (d), or left untreated (b). On day 25, the E749–57-specific CTL activity in those mice was measured using an in vivo CTL assay by injecting them via the tail vein with CFSElowand E749–57-labeled CFSEhighsplenocytes (10×106 each). Mice were sacrificed 13 h later, and their splenocyte suspension was prepared and analyzed by flow cytometry. One representative from three mice, which showed similar CTL activity, is shown. The experiment was repeated twice. Shown in a was the flow cytometry graph of naïve mice that were not seeded with TC-1 cells. Numbers shown above each region were the relative percent of CFSElow versus the CSFEhigh cells. A significant E749–57-specific CTL activity was detected only in tumor-bearing mice treated with pI:C/E749–57
Fig. 7
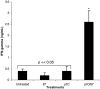
Splenocytes isolated from mice immunized with pI:C/E749–57 secreted IFN-γ after in vitro re-stimulation. Mice (_n_=3) were immunized with pI:C/E749–57, pI:C alone (50 μg), E749–57 alone (20 μg), or left untreated on days 0 and 7. On day 20, splenocytes were isolated from them, and co-incubated with E749–57(10 μg/1 × 107 cells) for 48 h. The concentration of IFN-γ in the culture medium was measured using ELISA. Data shown are mean ± S.E.M. (_n_=3). This experiment was repeated twice. Similar trend was observed. (asterisks) indicates that the result from pI:C/E749–57 was significantly different from that from other treatments (_P_=0.002 vs. untreated, t test). The values from mice treated with pI:C, E749–57, or left untreated are not different from each other
Fig. 8
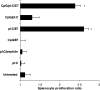
Splenocytes isolated from mice immunized with pI:C/E749–57 or pI:C/CpG/E749–57proliferated significantly after in vitro re-stimulation. Mice were seeded with TC-1 tumor cells (5×105) on day 0. On days 4 and 7, they were immunized with either pI:C alone, pI:C/E749–57, pI:C/9 aa peptide, CpG1826 admixed with E749–57, CpG1826 and pI:C mixture, pI:C/E749–57 admixed with CpG1826, or left untreated. The dose for peptide, pI:C, and CpG1826 was 20, 50, and 20 μg/mouse, respectively. On day 25, mice were euthanized; and their spleen was removed. Single splenocyte suspensions from each individual spleen were prepared and stimulated with E749–57 (0 or 100 μg/ml) for 5 days. The cell number was determined using an MTT test kit. asterisks indicates that the values from pI:C/E749–57 and pI:C/CpG/E749–57 were comparable, but significantly different from that of the other groups. This experiment was repeated twice. Similar trend was observed. Data shown are mean ± S.D. (_n_=5)
Fig. 9
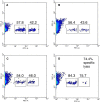
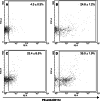
Poly(I:C) enhanced the proportion of DCs in local draining LNs. Mice (_n_=4) were injected s.c. into their hind leg footpads with pI:C (50 μg in 20 μl) (b), pI:C/E749–57 (c), LPS (5 μg in 20 μl) (d), or sterile PBS (10 mM, pH 7.4) (a). Draining popliteal LNs were removed 24 h after the injection. Single LN cell suspension was prepared from LNs, stained with PE-labeled anti-CD11c Ab, and analyzed by flow cytometry. Graphs shown are one representative from four mice. Numbers in the graphs are the percent of LN cells that were CD11c+(mean ± S.D., _n_=4)
Fig. 10
Immunization of tumor-bearing mice with pI:C/E749–57 restored the proportion of DCs in their LNs. Mice were s.c. injected with TC-1 cells (5×105) on day 0. On days 4 and 7, they were immunized with pI:C (d), pI:C/9 aa peptide (e), pI:C/E749–57(f), or left untreated (c). The dose of peptide was 20 μg/mouse; the dose of pI:C was 50 μg/mouse. LNs (popliteal, axillary, and inguinal) from five mice in each group were pooled. LN cells were stained with FITC-labeled anti-CD11c Ab and PE-labeled anti-CD86 Ab, and analyzed by flow cytometry. As a control, LNs from naïve mice (b) were also isolated and processed identically.Numbers in the graphs are the percent of CD11c+, CD86+double positive cells. This experiment was repeated twice. In both times, the percentages of CD11c+ and CD86+ double positive cells in c, d, ande were significantly lower than that inb and f
Similar articles
- Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma.
Ressing ME, van Driel WJ, Brandt RM, Kenter GG, de Jong JH, Bauknecht T, Fleuren GJ, Hoogerhout P, Offringa R, Sette A, Celis E, Grey H, Trimbos BJ, Kast WM, Melief CJ. Ressing ME, et al. J Immunother. 2000 Mar-Apr;23(2):255-66. doi: 10.1097/00002371-200003000-00010. J Immunother. 2000. PMID: 10746552 - A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive.
Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, Small LA, Kast WM, Fascio G, Marty V, Weber J. Muderspach L, et al. Clin Cancer Res. 2000 Sep;6(9):3406-16. Clin Cancer Res. 2000. PMID: 10999722 Clinical Trial. - A synthetic chimeric peptide harboring human papillomavirus 16 cytotoxic T lymphocyte epitopes shows therapeutic potential in a murine model of cervical cancer.
Sharma C, Khan MA, Mohan T, Shrinet J, Latha N, Singh N. Sharma C, et al. Immunol Res. 2014 Jan;58(1):132-8. doi: 10.1007/s12026-013-8447-2. Immunol Res. 2014. PMID: 24174302 - Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: therapeutic effect against cervical cancer.
Cui Z, Huang L. Cui Z, et al. Cancer Immunol Immunother. 2005 Dec;54(12):1180-90. doi: 10.1007/s00262-005-0685-2. Epub 2005 Apr 22. Cancer Immunol Immunother. 2005. PMID: 15846491 Free PMC article. - Clinical studies of human papilloma vaccines in pre-invasive and invasive cancer.
Adams M, Borysiewicz L, Fiander A, Man S, Jasani B, Navabi H, Lipetz C, Evans AS, Mason M. Adams M, et al. Vaccine. 2001 Mar 21;19(17-19):2549-56. doi: 10.1016/s0264-410x(00)00488-6. Vaccine. 2001. PMID: 11257391 Review.
Cited by
- TLR-mediated induction of negative regulatory ligands on dendritic cells.
Gröschel S, Piggott KD, Vaglio A, Ma-Krupa W, Singh K, Goronzy JJ, Weyand CM. Gröschel S, et al. J Mol Med (Berl). 2008 Apr;86(4):443-55. doi: 10.1007/s00109-008-0310-x. Epub 2008 Feb 6. J Mol Med (Berl). 2008. PMID: 18253710 Free PMC article. - Combinatorial treatment with polyI:C and anti-IL6 enhances apoptosis and suppresses metastasis of lung cancer cells.
Lau WH, Zhu XG, Ho SWT, Chang SC, Ding JL. Lau WH, et al. Oncotarget. 2017 May 16;8(20):32884-32904. doi: 10.18632/oncotarget.15862. Oncotarget. 2017. PMID: 28427199 Free PMC article. - HPV-Associated Tumor Eradication by Vaccination with Synthetic Short Peptides and Particle-Forming Liposomes.
He X, Zhou S, Quinn B, Jahagirdar D, Ortega J, Abrams SI, Lovell JF. He X, et al. Small. 2021 Mar;17(11):e2007165. doi: 10.1002/smll.202007165. Epub 2021 Feb 19. Small. 2021. PMID: 33605054 Free PMC article. - Polyinosinic-cytidylic acid as an adjuvant on natural killer- and dendritic cell-mediated antitumor activities.
Huang YK, Zheng Z, Qiu F. Huang YK, et al. Tumour Biol. 2013 Jun;34(3):1615-23. doi: 10.1007/s13277-013-0693-3. Epub 2013 Feb 22. Tumour Biol. 2013. PMID: 23430582 - Activation and inhibition of transglutaminase 2 in mice.
Dafik L, Albertelli M, Stamnaes J, Sollid LM, Khosla C. Dafik L, et al. PLoS One. 2012;7(2):e30642. doi: 10.1371/journal.pone.0030642. Epub 2012 Feb 2. PLoS One. 2012. PMID: 22319575 Free PMC article.
References
- Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63(12):3281–3288. - PubMed
- Davila E, Celis E. Repeated administration of cytosine-phosphorothiolated guanine-containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J Immunol. 2000;165(1):539–547. - PubMed
- Miconnet I, Koenig S, Speiser D, et al. CpG are efficient adjuvants for specific CTL induction against tumor antigen-derived peptide. J Immunol. 2002;168(3):1212–1218. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous