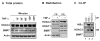Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function - PubMed (original) (raw)
Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function
Zhanguo Gao et al. J Biol Chem. 2006.
Abstract
Inhibition of peroxisome proliferator-activated receptor gamma (PPARgamma) function by TNF-alpha contributes to glucose and fatty acid metabolic disorders in inflammation and cancer, although the molecular mechanism is not fully understood. In this study, we demonstrate that nuclear translocation of HDAC3 is regulated by TNF-alpha, and this event is required for inhibition of transcriptional activity of PPARgamma by TNF-alpha. HDAC3 is associated with IkappaBalpha in the cytoplasm. After IkappaBalpha degradation in response to TNF-alpha, HDAC3 is subject to nuclear translocation, leading to an increase in HDAC3 activity in the nucleus. This event leads to subcellular redistribution of HDAC3. Knock-out of IkappaBalpha, but not p65 or p50, leads to disappearance of HDAC3 in the cytoplasm, which is associated with HDAC3 enrichment in the nucleus. These data suggest that inhibition of PPARgamma by TNF-alpha is not associated with a reduction in the DNA binding activity of PPARgamma. Rather, these results suggest that IkappaBalpha-dependent nuclear translocation of HDAC3 is responsible for PPARgamma inhibition by TNF-alpha.
Figures
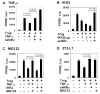
Fig. 1. Inhibition of PPARγ reporter activity by TNF-α
The transcriptional activity of PPARγ was analyzed in HEK293 cells and 3T3-L1 fibroblasts using the PPRE(3x)-luciferase reporter system in the transient transfection. The transfection and data analysis were performed as stated in the methods. (A) Inhibition of TNF-α activity by ssIkB in HEK293 cells. The reporter activity was induced with Troglitazone (Trog, 1 μM). TNF-α treatment was conducted at a final concentration of 10 ng/ml unless indicated specifically. (B) Inhibition of IKK activity by ssIkBα in HEK293 cells. (C) Inhibition of TNF-α and IKK activities by proteasome inhibitor MG-132 at a final concentration of 15 μM. (D) Inhibition of TNF-α activity by ssIkBα or MG-132 in 3T3-L1 adipocytes.
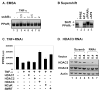
Fig. 2. HDAC3 and SMRT in TNF-induced PPARγ inhibition
(A) EMSA for DNA-binding activity of PPARγ. The assay was done with the nuclear extract of HEK293 cells that were transfected with the PPARγ2 expression vector. ssIkBα expression vector was co-transfected at dosages (μg) indicated at the top of each lane. The nuclear extract was made after two hour treatment with TNF-α (10 ng/ml). (B) Confirmation of PPARγ-DNA complex in supershift assay. (C) Analysis of corepressors with RNAi-mediated gene knockdown in the PPARγ reporter assay. RNAi-expression vectors for HDAC1-3, NCoR and SMRT were co-transfected with the reporter system into HEK293 cells at 0.2 μg/point. The reporter was induced by Troglitazone (Trog) and inhibited by TNF-α. (D) Test of RNAi effect on HDAC3 expression in an immunoblot. HDAC3 protein level was determined in HEK293 cells transfected with control vector or RNAi expression vector. The dosage (μg) of each vector was shown at the top of each lane.
Fig. 3. HDAC3 interaction with PPARγ in adipocytes
(A) ChIP assay for the interaction of HDAC3 and PPARγ in aP2 gene promoter. The assay was conducted in 3T3-L1 mature adipocytes after TNF-α treatment (10 ng/ml, 30 minutes). (B) DNA-binding activity of PPARγ in EMSA. The nuclear extract was made from the mature 3T3-L1 adipocytes after TNF-α treatment at different times as indicated at the top of each lane. Unlabelled PPRE probe or AP-1 probe was used in oligo competition. In these two experiments, Troglitazone (Trog) was used at a final concentration of 1 μM for 24 hour treatment.
Fig. 4. Nuclear translocation of HDAC3 induced by TNF-α
**(A)**The total protein of HDAC3 in 3T3-L1 adipocytes. The mature 3T3-L1 adipocytes were serum-starved overnight and treated with TNF-α for different times as indicated. The whole cell lysate was made and used in the immunoblot. (B) Immunoblot of the cytoplasmic and nuclear extracts of mature 3T3-L1 adipocytes. The cytoplasmic (EC) and nuclear (NE) extracts were made as stated in the experimental procedure. The protein abundance of HDAC3 and SMRT was examined in an immunoblot. HDAC1 and actin were controls in the nuclear and cytoplasmic extracts, respectively. (C) Association of IkBα and HDAC3 in coimmunoprecipitation. The cytoplasmic extract was made from 3T3-L1 adipocytes and used in the Co-IP. IgG was a control for non-specific signal.
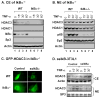
Fig. 5. Regulation of nuclear translocation of HDAC3 by IkBα
(A) Immunoblot of the cytoplasmic extract of wild type (WT) and IkBα−/− MEFs. The cells were treated with TNF-α for 15 minutes as indicated. HDAC3 protein was determined in the cytoplasmic extract in an immunoblot. HDAC1 and Sp3 were used as negative controls since they are nuclear proteins. IkBα and actin were positive controls in the cytoplasmic extract. (B) Immunoblot of the nuclear extract of wild type and IkBα−/− MEFs. HDAC1, p65 and Sp3 were positive controls in the nuclear extract. Actin was a negative control. (C) Intracellular distribution of GFP-HDAC3 in WT and IkBα−/− MEFs. The cells were transiently transfected with expression vectors for GFP-HDAC3 and ssIkBα. The pictures were made 48 hour later under a fluorescence microscope with oil lens (100 X). (D) Immunoblot of the cytoplasmic (CE) and nuclear extracts (CE) from the control and ssIkBα cell lines.
Fig. 6. Inhibition of TNF-α activity by ssIkBα in 3T3-L1 adipocytes
(A) Lipid droplet in adipocytes from the control and ssIkBα cells. The picture was made at day 7 of differentiation under a microscope at 400 times of magnification. The large lipid droplets are highlighted by the arrows. (B) Molecular markers of adipocytes in immunoblot. The cells were treated with TNF-α during adipogenesis. Adipogenesis was determined by the adipocyte-specific markers in the whole cell lysate at day 7 and 8 of differentiation. The markers include adiponectin (ApN), fatty acid binding protein 4 (aP2), PPARγ and glucose transporter 4 (GLUT4). (C) HDAC3-PPARγ interaction in adipocytes. Mature adipocytes were obtained by differentiation of 3T3-L1 fibroblasts in the adipogenic cocktail for 7 days. ChIP assay was conducted to determine the interaction in adipocytes after TNF-α treatment for 30 minutes. The cells were serum-starved for 4 hours before exposure to TNF-α (10 ng/ml). (D) Inhibition of aP2 mRNA expression by TNF-α in mature adipocytes. mRNA of aP2 was determined by quantative real time RT-PCR in 3T3-L1 adipocytes after overnight TNF-α treatment.
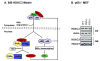
Fig. 7. Regulation of HDAC3 cytoplasm-nucleus shuttling by IkBα
(A) IkB-HDAC3 model. HDAC3 stays in the cytoplasm through association with IkBα. After IkBα degradation, HDAC3 enters the nucleus where it inhibits the transcriptional activity of PPARγ through histone deacetylation. When the newly-synthesized IkBα is available, it associates with nuclear HDAC3 and transfers HDAC3 into the cytoplasm. HDAC3 may enter and leave the nucleus through this mechanism. In this model, the role of IkBα is highlighted in the control of cytoplasm-nucleus shuttling of HDAC3. (B) HDAC3 distribution in p65−/− or p50−/− MEFs. HDAC3 abundance was determined in the cytoplasmic and nuclear extracts of the wild type and the knockout MEFs in an immunoblot.
Similar articles
- Regulation of PPARgamma function by TNF-alpha.
Ye J. Ye J. Biochem Biophys Res Commun. 2008 Sep 26;374(3):405-8. doi: 10.1016/j.bbrc.2008.07.068. Epub 2008 Jul 23. Biochem Biophys Res Commun. 2008. PMID: 18655773 Free PMC article. Review. - Effect of Salusin-β on Peroxisome Proliferator-Activated Receptor Gamma Gene Expression in Vascular Smooth Muscle Cells and its Possible Mechanism.
Xu X, He M, Liu T, Zeng Y, Zhang W. Xu X, et al. Cell Physiol Biochem. 2015;36(6):2466-79. doi: 10.1159/000430207. Epub 2015 Aug 10. Cell Physiol Biochem. 2015. PMID: 26279448 - Nuclear corepressor is required for inhibition of phosphoenolpyruvate carboxykinase expression by tumor necrosis factor-alpha.
Yan J, Gao Z, Yu G, He Q, Weng J, Ye J. Yan J, et al. Mol Endocrinol. 2007 Jul;21(7):1630-41. doi: 10.1210/me.2007-0072. Epub 2007 Apr 24. Mol Endocrinol. 2007. PMID: 17456789 - Inhibition of glyceroneogenesis by histone deacetylase 3 contributes to lipodystrophy in mice with adipose tissue inflammation.
Zhang J, Henagan TM, Gao Z, Ye J. Zhang J, et al. Endocrinology. 2011 May;152(5):1829-38. doi: 10.1210/en.2010-0828. Epub 2011 Mar 15. Endocrinology. 2011. PMID: 21406501 Free PMC article. - Duration of nuclear NF-kappaB action regulated by reversible acetylation.
Chen Lf, Fischle W, Verdin E, Greene WC. Chen Lf, et al. Science. 2001 Aug 31;293(5535):1653-7. doi: 10.1126/science.1062374. Science. 2001. PMID: 11533489
Cited by
- Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility.
Jain N, Iyer KV, Kumar A, Shivashankar GV. Jain N, et al. Proc Natl Acad Sci U S A. 2013 Jul 9;110(28):11349-54. doi: 10.1073/pnas.1300801110. Epub 2013 Jun 24. Proc Natl Acad Sci U S A. 2013. PMID: 23798429 Free PMC article. - Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes.
He Q, Yang QC, Zhou Q, Zhu H, Niu WY, Feng J, Wang Y, Cao J, Chen BY. He Q, et al. PLoS One. 2014 Jan 21;9(1):e86326. doi: 10.1371/journal.pone.0086326. eCollection 2014. PLoS One. 2014. PMID: 24466027 Free PMC article. - Regulation of PPARgamma function by TNF-alpha.
Ye J. Ye J. Biochem Biophys Res Commun. 2008 Sep 26;374(3):405-8. doi: 10.1016/j.bbrc.2008.07.068. Epub 2008 Jul 23. Biochem Biophys Res Commun. 2008. PMID: 18655773 Free PMC article. Review. - Regulation of insulin degrading enzyme activity by obesity-associated factors and pioglitazone in liver of diet-induced obese mice.
Wei X, Ke B, Zhao Z, Ye X, Gao Z, Ye J. Wei X, et al. PLoS One. 2014 Apr 16;9(4):e95399. doi: 10.1371/journal.pone.0095399. eCollection 2014. PLoS One. 2014. PMID: 24740421 Free PMC article. - Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation.
Pooladanda V, Thatikonda S, Bale S, Pattnaik B, Sigalapalli DK, Bathini NB, Singh SB, Godugu C. Pooladanda V, et al. Cell Death Dis. 2019 Jan 28;10(2):81. doi: 10.1038/s41419-018-1247-9. Cell Death Dis. 2019. PMID: 30692512 Free PMC article.
References
- Spiegelman BM. Diabetes. 1998;47(4):507–514. - PubMed
- Berger J, Moller DE. Annu Rev Med. 2002;53:409–435. - PubMed
- Lazar MA. Nat Med. 2001;7(1):23–24. - PubMed
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Science. 1996;274(5295):2100–2103. - PubMed
- Zhang B, Berger J, Hu E, Szalkowski D, White-Carrington S, Spiegelman BM, Moller DE. Mol Endocrinol. 1996;10(11):1457–1466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials