Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion - PubMed (original) (raw)
Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion
Rubén A Bartolomé et al. Cancer Res. 2006.
Abstract
Melanoma cells express the chemokine receptor CXCR4, which confers invasive signals on binding to its ligand CXCL12. We show here that knocking down membrane-type matrix metalloproteinase (MT1-MMP) expression translates into a blockade of invasion across reconstituted basement membranes and type I collagen gels in response to CXCL12, which is the result of lack of MMP-2 activation. Interference with MMP-2 expression further confirms its important role during this invasion. Vav proteins are guanine-nucleotide exchange factors for Rho GTPases that regulate actin dynamics and gene expression. We show that melanoma cells express Vav1 and Vav2, which are activated by CXCL12 involving Jak activity. Blocking Vav expression by RNA interference results in impaired activation of Rac and Rho by CXCL12 and in a remarkable inhibition of CXCL12-promoted invasion. Importantly, up-regulation of MT1-MMP expression by CXCL12, a mechanism contributing to melanoma cell invasion, is blocked by knocking down Vav expression or by inhibiting Jak. Together, these data indicate that activation of Jak/Vav/Rho GTPase pathway by CXCL12 is a key signaling event for MT1-MMP/MMP-2-dependent melanoma cell invasion.
Figures
Figure 1
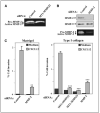
CXCR4 and MT1-MMP requirement for CXCL12-promoted melanoma cell invasion. A, BLM melanoma cells were transfected with siRNA for CXCR4 or with two different siRNAs for MT1-MMP (2 and 3), and transfectants were subjected to invasion assays across Matrigel toward CXCL12 or invasion medium alone. Columns, mean % cell invasion of three independent experiments done in duplicate; bars, SD. ***, P < 0.001; **, P < 0.01, invasion was significantly inhibited (one-way ANOVA test). Middle and right, expression of CXCR4 and MT1-MMP on BLM siRNA transfectants was analyzed by flow cytometry and Western blotting. Blots were reprobed with anti-paxillin mAb to check for total protein content. B, BLM cells were transfected with pEGFP (GFP) or GFP-fused WT MT1-MMP (MT1-GFP) expression vectors and analyzed by confocal microscopy (left) or subjected to invasion assays toward CXCL12 ( right). Columns, mean % invasive GFP-positive cells of two independent experiments done in duplicate; bars, SD. **, P < 0.01, invasion was significantly stimulated with respect to GFP transfectants (one-way ANOVA test).
Figure 2
Role of MMP-2 on CXCL12-promoted melanoma cell invasion. A, supernatants from BLM cells transfected with control or MT1-MMP (2) siRNA were analyzed by gelatin zymography. B, BLM cells were transfected with MMP-2 or control siRNA, and transfectant lysates were subjected to Western blotting using anti-MMP-2 mAb followed by reprobing membranes with anti-MMP-9 antibodies (top and middle). Supernatants from transfected cells were assayed by gelatin zymography to detect MMP-2 (bottom). C, control, MT1-MMP, or MMP-2 siRNA BLM transfectants were subjected to invasion assays across Matrigel (left) or type I collagen gels (right) toward CXCL12 or medium alone. Columns, mean % cell invasion of three (B) or two (C) independent experiments done in duplicate; bars, SD. ***, P < 0.001; **, P < 0.01, invasion was significantly inhibited (one-way ANOVA test).
Figure 3
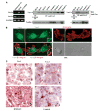
Expression of Vav proteins on melanoma cells. A, lysates from metastatic melanoma cells isolated from infiltrated lymph nodes (Melanoma LN) and BLM melanoma cells were analyzed by RT-PCR using Vav1- or Vav2-specific primers. Lysates from NCI-H929 myeloma cells were used as positive controls. Control amplification using GAPDH-specific primers (left). Cell lysates from the melanoma cell lines BLM, Mel 57, and MeWo and from four different metastatic melanoma samples (Sample 1-Sample 4) were subjected to immunoprecipitation followed by Western blotting using Vav1, Vav2, or control antibodies. Aliquots from cell lysates were kept aside for subsequent loading control in Western blotting using anti-paxillin antibodies (middle and right). B, BLM cells were subjected to confocal microscopy using anti-Vav1, anti-Vav2, or anti-integrin β1 antibodies followed by FITC- or Texas red–conjugated secondary antibodies. Merged Vav1/β1 and Vav2/β1 as well as 5-(3,3-dimethyl-triazeno) imidazole-4-carboxamide differential interference contrast (DIC) microscopy images are also shown. Insets, enlargements of membrane protrusion structures. Control antibodies gave no staining (data not shown). C, immunohistochemical analysis of Vav1 and Vav2 expression on melanoma lymph node metastasis. Original magnification, x400. a, anti-Vav1; b, anti-Vav2; c, anti-HMB-45; d, control rabbit serum. Note in (c) the predominant red staining of melanoma cells with the selective anti-HMB-45 antibody as well as red positive staining of tumor cells by Vav1 (a) and Vav2 (b) antibodies. Brown color is due to the melanin content of tumor cells.
Figure 4
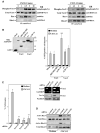
Role of Vav proteins on CXCL12-promoted melanoma cell invasion. A, BLM cells were incubated for the indicated times with CXCL12 followed by cell lysis and immunoprecipitation with anti-Vav1 or Vav-2 antibodies. Immunoprecipitates were resolved by SDS-PAGE and proteins were electrotransferred to PVDF membranes that were incubated sequentially with anti-phosphotyrosine, anti-Vav1 or anti-Vav-2, anti-Rac, and anti-RhoA antibodies. B, BLM cells were transfected with expression vectors coding for GFP-fused WT or the indicated mutant Vav1 or Vav2 or GFP alone (Mock), and trans-fectants were lysed and subjected to Western blotting using anti-GFP antibodies (left) or to Matrigel invasion assays in response to CXCL12 (right). C, left, BLM cells were transfected with control, Vav1, or Vav2 siRNA, and transfectants were tested in Matrigel invasion assays to CXCL12; right, BLM siRNA transfectants were analyzed by RT-PCR and Western blotting using Vav1- or Vav2-specific reagents. D, BLM siRNA transfectants were incubated with or without CXCL12 and subjected to GTPase assays to detect active Rac and RhoA. Columns, mean % cell invasion of at least three independent experiments done in duplicate; bars, SD. **, P < 0.01, invasion was significantly inhibited (one-way ANOVA test); ΔΔ, P < 0.01, invasion was significantly increased in comparison with invasion displayed by Vav1 WT transfectants incubated with CXCL12.
Figure 5
Role of Jak on CXCL12-promoted melanoma cell invasion and Vav activation. A, BLM cells were subjected to Matrigel invasion assays to CXCL12 in the absence or presence of the indicated concentrations of AG490. Columns, mean % cell invasion of three independent experiments done in duplicate; bars, SD. ***, P < 0.001; *, P < 0.05, invasion was significantly inhibited (one-way ANOVA test). B, BLM cells treated without (Control) or with AG490 (2 hours, 25 μmol/L) were incubated for 5 minutes in the absence or presence of CXCL12 (150 ng/mL) and subjected to analyses of Vav1 or Vav2 phosphorylation as described in Fig. 4.
Figure 6
Vav controls up-regulation of MT1-MMP expression in response to CXCL12. BLM cells transfected with Vav1 (3), Vav2 (2), or control siRNA (A) or untransfected BLM cells treated with or without AG490 (B) were incubated for 24 hours with or without CXCL12, and following solubilization, cell lysates were analyzed by Western blotting using anti-MT1-MMP mAb. Blots were reprobed with anti-paxillin mAb to test for sample protein content. Bottom, densitometer analysis in arbitrary units showing fold induction of MT1-MMP expression from above gels.
Figure 7
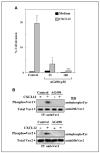
Role of PI3K in CXCL12-promoted melanoma cell invasion. A, BLM cells were incubated with CXCL12 (150 ng/mL) for the indicated times, and following solubilization, cell lysates were analyzed by Western blotting using anti-phospho-Akt followed by reprobing with anti-Akt antibodies. B, BLM cells were subjected to Matrigel invasion assays toward CXCL12 in the absence (Control) or in the presence of 25 μmol/L LY294002 or 100 nmol/L wortmannin. Columns, mean % cell invasion of three independent experiments done in duplicate; bars, SD. **, P < 0.01, invasion was significantly inhibited (one-way ANOVA test). C, BLM cells were incubated with or without CXCL12 in the absence or presence of LY294002 or wortmannin. Following solubilization, cell lysates were analyzed by Western blotting using anti-MT1-MMP mAb. Blots were reprobed with anti-paxillin mAb to test for protein content. D, control, Vav1 (3), or Vav2 (2) siRNA BLM transfectants were incubated for 5 minutes with or without CXCL12 and subjected to Western blot analyses of phospho-Akt and total Akt expression as above.
References
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. -PubMed
- Robledo MM, Bartolome RA, Longo N, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–105. -PubMed
- Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. -PubMed
- Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–9. -PubMed
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. -PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous