Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? - PubMed (original) (raw)
Clinical Trial
. 2006 Jan 30;94(2):259-67.
doi: 10.1038/sj.bjc.6602930.
M Gelly, F Penault-Llorca, L Benoit, F Bonnetain, C Migeon, V Cabaret, V Fermeaux, P Bertheau, J Garnier, J-F Jeannin, B Coudert
Affiliations
- PMID: 16404427
- PMCID: PMC2361112
- DOI: 10.1038/sj.bjc.6602930
Clinical Trial
Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism?
L Arnould et al. Br J Cancer. 2006.
Abstract
This study evaluated by immunohistochemistry (IHC) immune cell response during neoadjuvant primary systemic therapy (PST) with trastuzumab in patients with HER2-positive primary breast cancer. In all, 23 patients with IHC 3+ primary breast cancer were treated with trastuzumab plus docetaxel. Pathological complete and partial responses were documented for nine (39%) and 14 (61%) patients, respectively. Case-matched controls comprised patients treated with docetaxel-based PST without trastuzumab (D; n=23) or PST without docetaxel or trastuzumab (non-taxane, non-trastuzumab, NT-NT; n=23). All surgical specimens were blind-analysed by two independent pathologists, with immunohistochemical evaluation of B and T lymphocytes, macrophages, dendritic cells and natural killer (NK) cells. Potential cytolytic cells were stained for Granzyme B and TiA1. HER2 expression was also evaluated in residual tumour cells. Trastuzumab treatment was associated with significantly increased numbers of tumour-associated NK cells and increased lymphocyte expression of Granzyme B and TiA1 compared with controls. This study supports an in vivo role for immune (particularly NK cell) responses in the mechanism of trastuzumab action in breast cancer. These results suggest that trastuzumab plus taxanes lead to enhanced NK cell activity, which may partially account for the synergistic activity of trastuzumab and docetaxel in breast cancer.
Figures
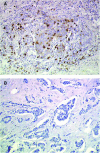
Figure 1
Immunohistochemical staining with NK1. Residual tumour from the TAXHER01 group (A) and from a matched tumour treated in the control group (B), both stained with NK1. The tumours of the TAXHER01 group show more cells in contact with or close to the tumour cells.
Figure 2
Immunohistochemical staining with CD56. Residual tumour from the TAXHER01 group (A) and from a matched tumour treated in the control group (B) both stained with CD56. The tumours of the TAXHER01 group show more cells in contact with or close to the tumour cells.
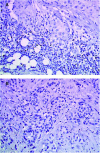
Figure 3
Immunohistochemical staining with Granzyme B. Residual tumour from the TAXHER01 group (A) and from a matched tumour treated in the control group (B), both stained with Granzyme B. The tumours of the TAXHER01 group show more cells in contact with or close to the tumour cells.
Figure 4
Immunohistochemical staining with TiA1. Residual tumour from the TAXHER01 group (A) and from a matched tumour treated in the control group (B), both stained with TiA1. The tumours of the TAXHER01 group show more cells in contact with or close to the tumour cells.
Comment in
- Administration of anti-HER2 antibody after nonmyeloablative allogeneic stem cell transplantation in metastatic breast cancer.
Banna GL, Aversa SM, Crivellari G, Ghiotto C, Chiarion-Sileni V, Monfardini S. Banna GL, et al. Br J Cancer. 2006 May 22;94(10):1550-2. doi: 10.1038/sj.bjc.6603114. Br J Cancer. 2006. PMID: 16641905 Free PMC article. No abstract available.
Similar articles
- T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival.
Ladoire S, Arnould L, Mignot G, Apetoh L, Rébé C, Martin F, Fumoleau P, Coudert B, Ghiringhelli F. Ladoire S, et al. Br J Cancer. 2011 Jul 26;105(3):366-71. doi: 10.1038/bjc.2011.261. Epub 2011 Jul 12. Br J Cancer. 2011. PMID: 21750556 Free PMC article. - Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial.
Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, Harbeck N, Valero V, Stroyakovskiy D, Wildiers H, Campone M, Boileau JF, Beckmann MW, Afenjar K, Fresco R, Helms HJ, Xu J, Lin YG, Sparano J, Slamon D. Hurvitz SA, et al. Lancet Oncol. 2018 Jan;19(1):115-126. doi: 10.1016/S1470-2045(17)30716-7. Epub 2017 Nov 23. Lancet Oncol. 2018. PMID: 29175149 Clinical Trial. - Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER): an open-label, randomised phase 2 trial.
Coudert B, Pierga JY, Mouret-Reynier MA, Kerrou K, Ferrero JM, Petit T, Kerbrat P, Dupré PF, Bachelot T, Gabelle P, Giard S, Coeffic D, Bougnoux P, Prevost JB, Paintaud G, Thibault G, Hernandez J, Coudert M, Arnould L, Berriolo-Riedinger A. Coudert B, et al. Lancet Oncol. 2014 Dec;15(13):1493-1502. doi: 10.1016/S1470-2045(14)70475-9. Epub 2014 Oct 30. Lancet Oncol. 2014. PMID: 25456368 Clinical Trial. - HER2-positive breast cancer: update on Breast Cancer International Research Group trials.
Nabholtz JM, Reese DM, Lindsay MA, Riva A. Nabholtz JM, et al. Clin Breast Cancer. 2002 Oct;3 Suppl 2:S75-9. doi: 10.3816/cbc.2002.s.016. Clin Breast Cancer. 2002. PMID: 12435291 Review. - Pertuzumab: a review of its use for first-line combination treatment of HER2-positive metastatic breast cancer.
McCormack PL. McCormack PL. Drugs. 2013 Sep;73(13):1491-502. doi: 10.1007/s40265-013-0109-0. Drugs. 2013. PMID: 23982598 Review.
Cited by
- Immunotherapy for Breast Cancer Treatment.
Simonian M, Haji Ghaffari M, Negahdari B. Simonian M, et al. Iran Biomed J. 2021 Mar 8;25(3):140-56. doi: 10.29252/ibj.25.3.140. Online ahead of print. Iran Biomed J. 2021. PMID: 33724757 Free PMC article. - A single proteolytic cleavage within the lower hinge of trastuzumab reduces immune effector function and in vivo efficacy.
Fan X, Brezski RJ, Fa M, Deng H, Oberholtzer A, Gonzalez A, Dubinsky WP, Strohl WR, Jordan RE, Zhang N, An Z. Fan X, et al. Breast Cancer Res. 2012 Aug 8;14(4):R116. doi: 10.1186/bcr3240. Breast Cancer Res. 2012. PMID: 22873525 Free PMC article. - Therapeutic Silencing of BCL-2 Using NK Cell-Derived Exosomes as a Novel Therapeutic Approach in Breast Cancer.
Kaban K, Hinterleitner C, Zhou Y, Salva E, Kantarci AG, Salih HR, Märklin M. Kaban K, et al. Cancers (Basel). 2021 May 15;13(10):2397. doi: 10.3390/cancers13102397. Cancers (Basel). 2021. PMID: 34063475 Free PMC article. - miRNAs as biomarkers of therapeutic response to HER2-targeted treatment in breast cancer: A systematic review.
Vo TH, El-Sherbieny Abdelaal E, Jordan E, O'Donovan O, McNeela EA, Mehta JP, Rani S. Vo TH, et al. Biochem Biophys Rep. 2023 Nov 28;37:101588. doi: 10.1016/j.bbrep.2023.101588. eCollection 2024 Mar. Biochem Biophys Rep. 2023. PMID: 38088952 Free PMC article. Review. - Overcoming resistance to PD-1/PD-L1 inhibitors in esophageal cancer.
Cheng C, Zhuge L, Xiao X, Luan S, Yuan Y. Cheng C, et al. Front Oncol. 2022 Sep 5;12:955163. doi: 10.3389/fonc.2022.955163. eCollection 2022. Front Oncol. 2022. PMID: 36132136 Free PMC article. Review.
References
- Baselga J, Gianni L, Geyer C, Perez EA, Riva A, Jackisch C (2004) Future options with trastuzumab for primary systemic and adjuvant therapy. Semin Oncol 31: 51–57 - PubMed
- Bines J, Murad A, Lago S, Ferrari B, Andrade J, Filho EA (2003) Weekly docetaxel (Taxotere®) and trastuzumab (Herceptin®) as primary therapy in stage III HER-2 overexpressing breast cancer – a Brazilian multicenter study. Breast Cancer Res Treat 82(Supplement 1): S56 (abstract 243)
- Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, Parker LM, Ellisen LW, Kuter I, Gadd MA, Christian RL, Kennedy PR, Borges VF, Bunnell CA, Younger J, Smith BL, Winer EP (2003) Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol 21: 46–53 - PubMed
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23: 3676–3685 - PubMed
- Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA (2001) Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol 31: 3016–3025 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous