Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes - PubMed (original) (raw)
Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes
Xifeng Wu et al. Am J Hum Genet. 2006 Mar.
Abstract
The candidate-gene approach in association studies of polygenic diseases has often yielded conflicting results. In this hospital-based case-control study with 696 white patients newly diagnosed with bladder cancer and 629 unaffected white controls, we applied a multigenic approach to examine the associations with bladder cancer risk of a comprehensive panel of 44 selected polymorphisms in two pathways, DNA repair and cell-cycle control, and to evaluate higher-order gene-gene interactions, using classification and regression tree (CART) analysis. Individually, only XPD Asp312Asn, RAG1 Lys820Arg, and a p53 intronic SNP exhibited statistically significant main effects. However, we found a significant gene-dosage effect for increasing numbers of potential high-risk alleles in DNA-repair and cell-cycle pathways separately and combined. For the nucleotide-excision repair pathway, compared with the referent group (fewer than four adverse alleles), individuals with four (odds ratio [OR] = 1.52, 95% CI 1.05-2.20), five to six (OR = 1.81, 95% CI 1.31-2.50), and seven or more adverse alleles (OR = 2.50, 95% CI 1.69-3.70) had increasingly elevated risks of bladder cancer (P for trend <.001). Each additional adverse allele was associated with a 1.21-fold increase in risk (95% CI 1.12-1.29). For the combined analysis of DNA-repair and cell-cycle SNPs, compared with the referent group (<13 adverse alleles), the ORs for individuals with 13-15, 16-17, and >or=18 adverse alleles were 1.22 (95% CI 0.84-1.76), 1.57 (95% CI 1.05-2.35), and 1.77 (95% CI 1.19-2.63), respectively (P for trend = .002). Each additional high-risk allele was associated with a 1.07-fold significant increase in risk. In addition, we found that smoking had a significant multiplicative interaction with SNPs in the combined DNA-repair and cell-cycle-control pathways (P<.01). All genetic effects were evident only in "ever smokers" (persons who had smoked >or=100 cigarettes) and not in "never smokers." A cross-validation statistical method developed in this study confirmed the above observations. CART analysis revealed potential higher-order gene-gene and gene-smoking interactions and categorized a few higher-risk subgroups for bladder cancer. Moreover, subgroups identified with higher cancer risk also exhibited higher levels of induced genetic damage than did subgroups with lower risk. There was a significant trend of higher numbers of bleomycin- and benzo[a]pyrene diol-epoxide (BPDE)-induced chromatid breaks (by mutagen-sensitivity assay) and DNA damage (by comet assay) for individuals in higher-risk subgroups among cases of bladder cancer in smokers. The P for the trend was .0348 for bleomycin-induced chromosome breaks, .0036 for BPDE-induced chromosome breaks, and .0397 for BPDE-induced DNA damage, indicating that these higher-order gene-gene and gene-smoking interactions included SNPs that modulated repair and resulted in diminished DNA-repair capacity. Thus, genotype/phenotype analyses support findings from CART analyses. This is the first comprehensive study to use a multigenic analysis for bladder cancer, and the data suggest that individuals with a higher number of genetic variations in DNA-repair and cell-cycle-control genes are at an increased risk for bladder cancer, confirming the importance of taking a multigenic pathway-based approach to risk assessment.
Figures
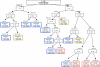
Figure 1
Classification and regression tree analysis of all the DNA-repair and cell-cycle gene polymorphisms. High-risk groups are identified by red-colored boxes, medium-risk groups by brown-colored boxes, and low-risk groups by blue-colored boxes.
Similar articles
- High-order interactions among genetic polymorphisms in nucleotide excision repair pathway genes and smoking in modulating bladder cancer risk.
Chen M, Kamat AM, Huang M, Grossman HB, Dinney CP, Lerner SP, Wu X, Gu J. Chen M, et al. Carcinogenesis. 2007 Oct;28(10):2160-5. doi: 10.1093/carcin/bgm167. Epub 2007 Aug 29. Carcinogenesis. 2007. PMID: 17728339 - High-order interactions among genetic variants in DNA base excision repair pathway genes and smoking in bladder cancer susceptibility.
Huang M, Dinney CP, Lin X, Lin J, Grossman HB, Wu X. Huang M, et al. Cancer Epidemiol Biomarkers Prev. 2007 Jan;16(1):84-91. doi: 10.1158/1055-9965.EPI-06-0712. Cancer Epidemiol Biomarkers Prev. 2007. PMID: 17220334 - Mutagen sensitivity and genetic variants in nucleotide excision repair pathway: genotype-phenotype correlation.
Lin J, Swan GE, Shields PG, Benowitz NL, Gu J, Amos CI, de Andrade M, Spitz MR, Wu X. Lin J, et al. Cancer Epidemiol Biomarkers Prev. 2007 Oct;16(10):2065-71. doi: 10.1158/1055-9965.EPI-06-1041. Cancer Epidemiol Biomarkers Prev. 2007. PMID: 17932354 - Molecular epidemiology of DNA repair genes in bladder cancer.
Kiltie AE. Kiltie AE. Methods Mol Biol. 2009;472:281-306. doi: 10.1007/978-1-60327-492-0_12. Methods Mol Biol. 2009. PMID: 19107438 Review. - rs1495741 as a tag single nucleotide polymorphism of N-acetyltransferase 2 acetylator phenotype associates bladder cancer risk and interacts with smoking: A systematic review and meta-analysis.
Ma C, Gu L, Yang M, Zhang Z, Zeng S, Song R, Xu C, Sun Y. Ma C, et al. Medicine (Baltimore). 2016 Aug;95(31):e4417. doi: 10.1097/MD.0000000000004417. Medicine (Baltimore). 2016. PMID: 27495060 Free PMC article. Review.
Cited by
- Association between APE1 Asp148Glu polymorphism and the risk of urinary cancers: a meta-analysis of 18 case-control studies.
Zhong JH, Zhao Z, Liu J, Yu HL, Zhou JY, Shi R. Zhong JH, et al. Onco Targets Ther. 2016 Mar 15;9:1499-510. doi: 10.2147/OTT.S101456. eCollection 2016. Onco Targets Ther. 2016. PMID: 27042118 Free PMC article. - Polymorphisms in the XPC gene affect urinary bladder cancer risk: a case-control study, meta-analyses and trial sequential analyses.
Sankhwar M, Sankhwar SN, Bansal SK, Gupta G, Rajender S. Sankhwar M, et al. Sci Rep. 2016 Jun 1;6:27018. doi: 10.1038/srep27018. Sci Rep. 2016. PMID: 27246180 Free PMC article. - A systematic review and meta-analysis of the association between OGG1 Ser326Cys polymorphism and cancers.
Zhou PT, Li B, Ji J, Wang MM, Gao CF. Zhou PT, et al. Med Oncol. 2015 Feb;32(2):472. doi: 10.1007/s12032-014-0472-z. Epub 2015 Jan 15. Med Oncol. 2015. PMID: 25588927 Review. - Information-theoretic gene-gene and gene-environment interaction analysis of quantitative traits.
Chanda P, Sucheston L, Liu S, Zhang A, Ramanathan M. Chanda P, et al. BMC Genomics. 2009 Nov 4;10:509. doi: 10.1186/1471-2164-10-509. BMC Genomics. 2009. PMID: 19889230 Free PMC article. - Genetic polymorphisms in double-strand break DNA repair genes associated with risk of oral premalignant lesions.
Yang H, Lippman SM, Huang M, Jack Lee J, Wang W, Spitz MR, Wu X. Yang H, et al. Eur J Cancer. 2008 Jul;44(11):1603-11. doi: 10.1016/j.ejca.2008.05.006. Epub 2008 Jun 23. Eur J Cancer. 2008. PMID: 18579371 Free PMC article.
References
Web Resources
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous