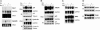Activated Src abrogates the Myc requirement for the G0/G1 transition but not for the G1/S transition - PubMed (original) (raw)
Activated Src abrogates the Myc requirement for the G0/G1 transition but not for the G1/S transition
Tulsiram Prathapam et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The transcription factor Myc plays a central role in the control of cellular proliferation. Myc expression is induced by growth factors in a pathway mediated by cellular Src (c-Src), but it is not clear whether Myc induction or activity is required for malignant transformation by activated Src. We introduced v-Src into a c-myc(-/-) derivative of Rat-1 fibroblasts and into 3T9 mouse fibroblasts harboring a conditionally excisable c-myc allele. Expression of activated viral Src in Myc-deficient cells led to loss of actin stress fibers and surface fibronectin, indicating that Myc is dispensable for v-Src-induced morphological transformation. However, v-Src failed to rescue the proliferative defect resulting from the loss of Myc. In Myc-deficient cells, despite its inability to overcome this proliferation block, v-Src was able to regulate the expression of certain Myc transcriptional targets and induce the expression of active cyclin D/Cdk4 and Cdk6 complexes; it also induced the phosphorylation of Rb, albeit at reduced levels. In contrast, however, in the absence of Myc, the level of Cdk2 kinase activity was drastically reduced. This reduction in Cdk2 activity was associated with a decrease in the expression of Cdk7, Cdc25A, and cyclin A. Coexpression of Cdk2 plus cyclin E and/or cyclin A rescued the G1/S block and allowed the cells to enter mitosis. These results indicate that in the absence of Myc, v-Src can activate early G1 cell cycle regulators but fails to activate regulators of the late G1/S transition.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
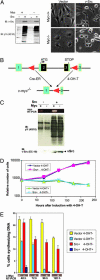
Effects of Myc deficiency on transformation of Rat1 and 3T9 cells by v-Src. (A) Morphological transformation of Myc-deficient Rat1 cells by v-Src. (A Left) Lysates of Rat1 myc+/+ and myc_−/− cells expressing either empty vector or v-Src were resolved by SDS/PAGE and immunoblotted with anti-Src mAb 2–17 or 4G10. (A Right) Phase contrast micrographs of same cells expressing either empty vector or v-Src. (B) Schematic showing the generation of Myc-deficient 3T9 cells by Cre-mediated myc excision. (C) Absence of Myc expression does not affect Src levels and activity. 3T9_mycflox/flox cells stably expressing Cre-ER and either empty vector or v-Src were induced with 4-OH-T to excise c-myc. Total RNA isolated from uninduced (Myc+) and 4-OH-T-induced (Myc−) cells was analyzed for myc mRNA levels by RT-PCR. Expression of v-Src and phosphotyrosine activity were analyzed by immunoblotting. (D) Expression of v-Src does not overcome the proliferative defect resulting from the deletion of c-myc. Equal numbers of 3T9_mycflox/flox_ cells stably expressing Cre-ER and either empty vector or v-Src were seeded in triplicate in medium containing 10% serum and induced with 4-OH-T for the indicated periods. Cells were trypsinized and counted with a Coulter counter. The data represent the number of cells relative to the number of cells at the time of plating (mean of three independent experiments). (E) v-Src expression does not rescue the defect in DNA synthesis resulting from the absence of Myc. Equal numbers of 3T9_mycflox/flox_ cells stably expressing Cre-ER and either empty vector or v-Src were induced with 4-OH-T for the indicated periods, and BrdUrd incorporation was assayed to determine the percentage of cells synthesizing DNA. Data represent the mean of three independent experiments ± SD. Myc levels were analyzed by RT-PCR.
Fig. 2.
Effects of Myc deficiency on the expression of Myc targets. Total RNA isolated from uninduced and 4-OH-T induced cells was analyzed by agarose gel electrophoresis and Northern hybridization, and RNA loading was monitored either by visualizing ribosomal RNAs or by Northern hybridization with a probe for GAPDH. Cell lysates were resolved by SDS/PAGE and protein expression monitored by immunoblotting, with either tubulin or GAPDH as loading control. (A) Expression of Gas1, Gadd45, p27, and cyclin D1. (B) Expression of nM23A and p21. (C) Expression of ODC and AIRC. (D) Expression of Cdk4 and Cdk6.
Fig. 3.
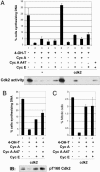
Effects of Myc deficiency on activation of cell cycle regulators by v-Src. (A) In the absence of Myc, v-Src induces cyclin D-dependent kinases but fails to activate Cdk2. In the top four blots, complexes were immunoprecipitated from extracts with antibodies indicated and kinase activities were assayed by using carboxyterminal Rb as substrate. In blots 5–7, cell lysates were analyzed by immunoblotting with anti-Rb antibody and phospho-specific Rb antibodies: _p_-T821 and _p_-S807 plus S811. (B) Failure of v-Src to induce active Cdk2 in c-_myc_−/− cells results from reduced expression of Cdk2, cyclin A, Cdk7, and Cdc25A. Equal amounts of lysates were analyzed by immunoblotting with antibodies directed against phospho T160-Cdk2, Cdk2, cyclins A and E, Cdk7, and Cdc25A. Cdk-activating enzyme/Cdk7 activity was measured by an immune complex kinase assay by using anti-Cdk7 antibody with recombinant Cdk2 protein as substrate. (C) The fraction of Cdk2 complexed to p21 is increased in the absence of Myc. p21 was immunodepleted from cell lysates by two successive rounds of immunoprecipitation with anti-p21 antibody, and coimmunoprecipitation of Cdk2 was monitored by immunoblotting. The supernatants obtained from the second round of p21 immunoprecipitation were again immunoprecipitated with anti-Cdk2 antibody to determine the levels of Cdk2 not bound to p21 (Bottom).
Fig. 4.
Cooverexpression of cyclins E and A and Cdk2 in v-Src transformed cells is able to rescue the G1/S block resulting from lack of Myc. 3T9_mycflox/flox_ Cre-ER v-Src-positive cells constitutively overexpressing Cdk2 were generated and then transiently transfected with expression plasmids as indicated: cyclin E, or cyclin A, both cyclin E and A, or cyclin E and a stabilized mutant of cyclin A (A47). Cells were induced with 4-OH-T to excise c-myc and BrdUrd incorporation was assayed to determine the percentage of cells synthesizing DNA. A and B represent two separate sets of experiments; for each set, the data represent the mean of three independent experiments ± SE. (A Lower) Cdk2 activity assayed as described in Fig. 3_A_. (B Lower) Immunoblotting with anti-phospho-T160-Cdk2 antibody. (C) Percentage of mitotic cells, obtained by anti-phospho-histone H3 staining.
Fig. 5.
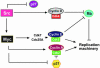
Schematic model depicting the significance of Myc in v-Src transformation. In the absence of Myc, v-Src regulates early cell cycle regulators such as p27 and cyclin D-Cdk4 but fails to induce the G1/S transition because of a failure to activate cyclin E/Cdk2 and cyclin A/Cdk complexes.
Similar articles
- Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F.
Leone G, DeGregori J, Sears R, Jakoi L, Nevins JR. Leone G, et al. Nature. 1997 May 22;387(6631):422-6. doi: 10.1038/387422a0. Nature. 1997. PMID: 9163430 - BCL-x(L) and BCL2 delay Myc-induced cell cycle entry through elevation of p27 and inhibition of G1 cyclin-dependent kinases.
Greider C, Chattopadhyay A, Parkhurst C, Yang E. Greider C, et al. Oncogene. 2002 Nov 7;21(51):7765-75. doi: 10.1038/sj.onc.1205928. Oncogene. 2002. PMID: 12420213 - Repression of c-Myc responsive genes in cycling cells causes G1 arrest through reduction of cyclin E/CDK2 kinase activity.
Berns K, Hijmans EM, Bernards R. Berns K, et al. Oncogene. 1997 Sep;15(11):1347-56. doi: 10.1038/sj.onc.1201280. Oncogene. 1997. PMID: 9315103 - Regulation of cell cycle entry and G1 progression by CSF-1.
Roussel MF. Roussel MF. Mol Reprod Dev. 1997 Jan;46(1):11-8. doi: 10.1002/(SICI)1098-2795(199701)46:1<11::AID-MRD3>3.0.CO;2-U. Mol Reprod Dev. 1997. PMID: 8981358 Review. - Making decisions through Myc.
Nasi S, Ciarapica R, Jucker R, Rosati J, Soucek L. Nasi S, et al. FEBS Lett. 2001 Feb 16;490(3):153-62. doi: 10.1016/s0014-5793(01)02118-4. FEBS Lett. 2001. PMID: 11223030 Review.
Cited by
- EZH2 synergizes with BRD4-NUT to drive NUT carcinoma growth through silencing of key tumor suppressor genes.
Huang Y, Durall RT, Luong NM, Hertzler HJ, Huang J, Gokhale PC, Leeper BA, Persky NS, Root DE, Anekal PV, Montero Llopis PDLM, David CN, Kutok JL, Raimondi A, Saluja K, Luo J, Zahnow CA, Adane B, Stegmaier K, Hawkins CE, Ponne C, Le Q, Shapiro GI, Lemieux ME, Eagen KP, French CA. Huang Y, et al. bioRxiv [Preprint]. 2023 Aug 16:2023.08.15.553204. doi: 10.1101/2023.08.15.553204. bioRxiv. 2023. PMID: 37645799 Free PMC article. Updated. Preprint. - EZH2 Cooperates with BRD4-NUT to Drive NUT Carcinoma Growth by Silencing Key Tumor Suppressor Genes.
Huang Y, Durall RT, Luong NM, Hertzler HJ, Huang J, Gokhale PC, Leeper BA, Persky NS, Root DE, Anekal PV, Montero Llopis PDLM, David CN, Kutok JL, Raimondi A, Saluja K, Luo J, Zahnow CA, Adane B, Stegmaier K, Hawkins CE, Ponne C, Le Q, Shapiro GI, Lemieux ME, Eagen KP, French CA. Huang Y, et al. Cancer Res. 2023 Dec 1;83(23):3956-3973. doi: 10.1158/0008-5472.CAN-23-1475. Cancer Res. 2023. PMID: 37747726 Free PMC article. - Src kinase function controls progenitor cell pools during regeneration and tumor onset in the Drosophila intestine.
Kohlmaier A, Fassnacht C, Jin Y, Reuter H, Begum J, Dutta D, Edgar BA. Kohlmaier A, et al. Oncogene. 2015 Apr 30;34(18):2371-84. doi: 10.1038/onc.2014.163. Epub 2014 Jun 30. Oncogene. 2015. PMID: 24975577 - The oncogene c-Myc coordinates regulation of metabolic networks to enable rapid cell cycle entry.
Morrish F, Neretti N, Sedivy JM, Hockenbery DM. Morrish F, et al. Cell Cycle. 2008 Apr 15;7(8):1054-66. doi: 10.4161/cc.7.8.5739. Epub 2008 Feb 8. Cell Cycle. 2008. PMID: 18414044 Free PMC article. - Newly synthesized 6-substituted piperazine/phenyl-9-cyclopentyl containing purine nucleobase analogs act as potent anticancer agents and induce apoptosis via inhibiting Src in hepatocellular carcinoma cells.
Bilget Guven E, Durmaz Sahin I, Altiparmak D, Servili B, Essiz S, Cetin-Atalay R, Tuncbilek M. Bilget Guven E, et al. RSC Med Chem. 2023 Nov 10;14(12):2658-2676. doi: 10.1039/d3md00440f. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38107180 Free PMC article.
References
- Abram C. L., Courtneidge S. A. Exp. Cell Res. 2000;254:1–13. - PubMed
- Welham M. J., Wyke J. A., Lang A., Wyke A. W. Oncogene. 1990;5:161–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA09041/CA/NCI NIH HHS/United States
- T32 GM007232/GM/NIGMS NIH HHS/United States
- GM07232/GM/NIGMS NIH HHS/United States
- CA17542/CA/NCI NIH HHS/United States
- R01 CA017542/CA/NCI NIH HHS/United States
- R37 CA017542/CA/NCI NIH HHS/United States
- T32 CA009041/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous