Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism - PubMed (original) (raw)
Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism
Ayyakannu Ayyanan et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Wnt and Notch signaling have long been established as strongly oncogenic in the mouse mammary gland. Aberrant expression of several Wnts and other components of this pathway in human breast carcinomas has been reported, but evidence for a causative role in the human disease has been missing. Here we report that increased Wnt signaling, as achieved by ectopic expression of Wnt-1, triggers the DNA damage response (DDR) and an ensuing cascade of events resulting in tumorigenic conversion of primary human mammary epithelial cells. Wnt-1-transformed cells have high telomerase activity and compromised p53 and Rb function, grow as spheres in suspension, and in mice form tumors that closely resemble medullary carcinomas of the breast. Notch signaling is up-regulated through a mechanism involving increased expression of the Notch ligands Dll1, Dll3, and Dll4 and is required for expression of the tumorigenic phenotype. Increased Notch signaling in primary human mammary epithelial cells is sufficient to reproduce some aspects of Wnt-induced transformation. The relevance of these findings for human breast cancer is supported by the fact that expression of Wnt-1 and Wnt-4 and of established Wnt target genes, such as Axin-2 and Lef-1, as well as the Notch ligands, such as Dll3 and Dll4, is up-regulated in human breast carcinomas.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
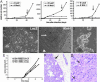
Fig. 1.
Effects of Wnt-1 on primary HMECs. (A) Growth curves of primary HMECs from three different individuals infected with Wnt-1- or LacZ-expressing retrovirus. Cell number relative to that at the time of infection is plotted over time. Wnt-1-HMEC strains continue to grow at 5 weeks after infection, whereas the LacZ-infected cells arrest. Arrows indicate when spheres start forming. (B and C) Micrographs of pLNCX-LacZ- and pLNCX-Wnt-1-infected HMECs 4 weeks after infection and selection (P5). pLNCX-LacZ-infected cells show hallmarks of senescence, whereas in pLNCX-Wnt-1 (P7)-infected cultures, small, rounded cells (arrows) appear next to flat, senescent cells (arrowheads). (Scale bars: 100 μm.) (D) More complex 3D structures formed by Wnt-1-HMECs 8 weeks after infections (P12). (Scale bar: 200 μm.) (E) Growth curves of tumors arising from three different Wnt-1-HMEC strains (P15–P20) injected into the mammary glands of _RAG2_−/− females. (F) Hematoxylin and eosin-stained sections of Wnt-1-HMEC tumors. Tumor cells grow as sheet and are surrounded by a pseudo capsule (arrow). (G) Higher magnification shows tumor cells with highly pleiomorphic nuclei and several mitotic figures (arrows).
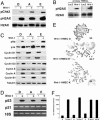
Fig. 2.
Hallmarks of oncogenic transformation in Wnt-1-HMECs. (A) Expression of the phosphorylated form of Chk-2 and H2AX protein as well as total H2AX protein levels in three different Wnt-1-HMEC strains and the respective parental cells. (B) Expression of the phosphorylated form of H2AX protein as well as total H2AX protein levels in primary HMECs (P5) from two different donors that were infected with retroviruses expressing either LacZ or Wnt-1. (C) Expression of different cell-cycle proteins assessed by immunoblotting of lysates from three different Wnt-1-HMEC strains and respective parental cells. Cyclin D1 and D2 levels are down-modulated in Wnt-1-HMEC strains, whereas p16, phospho Rb, cyclin D3, cyclin A, and cyclin B1 levels are up-regulated. Tubulin was used as a loading control. (D) p53 protein levels assessed by immunoblotting are high in Wnt-1-transformed cells and below detection limit in parental controls. p21 mRNA levels are comparable. Real-time PCR quantification (data not shown) indicates that p21 mRNA levels normalized with 18S rRNA do not differ in a statistically significant manner. (E) Representative mitotic spreads of different Wnt-1-HMEC strains showing near triploid karyotype; average chromosome number for each cell strain is indicated (n = 10). (F) Telomerase activity in different Wnt-1-HMEC strains, their parental control cells, and HeLa cells assessed by real-time quantitative telomeric repeat amplification protocol.
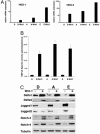
Fig. 3.
Notch signaling in Wnt-1-HMECs and in primary HMECs. (A) RT-PCR analysis of mRNA expression of the endogenous Notch target genes HES-1 and HES-5 in the parental HMECs and Wnt-1-HMECs. All real-time RT-PCR values are expressed as relative arbitrary units after internal normalization for 18S rRNA. (B) Notch signaling activity in different Wnt-1-HMECs (P15–P18) and parental HMECs (P4 and P5) derived from different reduction mammoplasties (D, A, and E) assayed with reporter plasmids containing an artificial Notch/RBP-Jk-responsive promoter (pGAwt) or a control reporter (pGAmut) without RBP-Jk-binding sites, plotted after internal normalization. (C) Immunoblot of Notch signaling components, Dll1, Dll4, Jagged1, and Jagged2, as well as Notch3 and Notch4 in parental HMECs (P4 and P5) and Wnt-1-HMECs (P15–P18) derived from different reduction mammoplasties (D, A, and E).
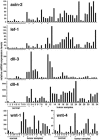
Fig. 4.
Axin-2, Lef-1, Dll-3, Dll-4, Wnt-1, and Wnt-4 mRNA expression levels in normal human breast tissue (n = 9 or 6) and breast carcinoma samples (n = 34 or 14) quantified by real-time RT-PCR. All samples were run in triplicate and normalized to 18S rRNA. To account for tumor-to-tumor variability in gene expression levels, each bar represents a single tumor. Asterisks indicate medullary carcinomas.
Similar articles
- Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas.
Hendrix ND, Wu R, Kuick R, Schwartz DR, Fearon ER, Cho KR. Hendrix ND, et al. Cancer Res. 2006 Feb 1;66(3):1354-62. doi: 10.1158/0008-5472.CAN-05-3694. Cancer Res. 2006. PMID: 16452189 - Wnt signalling in human breast cancer: expression of the putative Wnt inhibitor Dickkopf-3 (DKK3) is frequently suppressed by promoter hypermethylation in mammary tumours.
Veeck J, Bektas N, Hartmann A, Kristiansen G, Heindrichs U, Knüchel R, Dahl E. Veeck J, et al. Breast Cancer Res. 2008;10(5):R82. doi: 10.1186/bcr2151. Epub 2008 Sep 30. Breast Cancer Res. 2008. PMID: 18826564 Free PMC article. - Wnt-1 and int-2 mammary oncogene effects on the beta-catenin pathway in immortalized mouse mammary epithelial cells are not sufficient for tumorigenesis.
Hollmann CA, Kittrell FS, Medina D, Butel JS. Hollmann CA, et al. Oncogene. 2001 Nov 15;20(52):7645-57. doi: 10.1038/sj.onc.1204980. Oncogene. 2001. PMID: 11753642 - Notch signaling and breast cancer.
Reedijk M. Reedijk M. Adv Exp Med Biol. 2012;727:241-57. doi: 10.1007/978-1-4614-0899-4_18. Adv Exp Med Biol. 2012. PMID: 22399352 Review. - Notch signaling as a therapeutic target for breast cancer treatment?
Han J, Hendzel MJ, Allalunis-Turner J. Han J, et al. Breast Cancer Res. 2011 May 31;13(3):210. doi: 10.1186/bcr2875. Breast Cancer Res. 2011. PMID: 21672271 Free PMC article. Review.
Cited by
- Herbal Ingredients in the Prevention of Breast Cancer: Comprehensive Review of Potential Molecular Targets and Role of Natural Products.
Küpeli Akkol E, Bardakci H, Barak TH, Aschner M, Şeker Karatoprak G, Khan H, Hussain Y. Küpeli Akkol E, et al. Oxid Med Cell Longev. 2022 Aug 16;2022:6044640. doi: 10.1155/2022/6044640. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36017236 Free PMC article. Review. - Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway.
Ma X, Yan W, Dai Z, Gao X, Ma Y, Xu Q, Jiang J, Zhang S. Ma X, et al. Drug Des Devel Ther. 2016 Apr 18;10:1419-41. doi: 10.2147/DDDT.S102541. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27143851 Free PMC article. - Moving Breast Cancer Therapy up a Notch.
Mollen EWJ, Ient J, Tjan-Heijnen VCG, Boersma LJ, Miele L, Smidt ML, Vooijs MAGG. Mollen EWJ, et al. Front Oncol. 2018 Nov 20;8:518. doi: 10.3389/fonc.2018.00518. eCollection 2018. Front Oncol. 2018. PMID: 30515368 Free PMC article. Review. - LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy.
Liu CC, Prior J, Piwnica-Worms D, Bu G. Liu CC, et al. Proc Natl Acad Sci U S A. 2010 Mar 16;107(11):5136-41. doi: 10.1073/pnas.0911220107. Epub 2010 Mar 1. Proc Natl Acad Sci U S A. 2010. PMID: 20194742 Free PMC article. - From fly wings to targeted cancer therapies: a centennial for notch signaling.
Ntziachristos P, Lim JS, Sage J, Aifantis I. Ntziachristos P, et al. Cancer Cell. 2014 Mar 17;25(3):318-34. doi: 10.1016/j.ccr.2014.02.018. Cancer Cell. 2014. PMID: 24651013 Free PMC article. Review.
References
- Ellis I. O., Schnitt S. J., Sastre-Garau X., Bussolati G., Tarassoli F. A., Eusebi V., Peterse J. L., Mukai K., Tabár L., Jacquemeir J. In: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Tavassoli F., Devilee P., editors. Vol. 5. Lyon, France: International Agency for Research on Cancer; 2003. pp. 13–59.
- Hanahan D., Weinberg R. A. Cell. 2000;100:57–70. - PubMed
- Cardiff R. D., Bern H. A., Faulkin L. J., Daniel C. W., Smith G. H., Young L. J., Medina D., Gardner M. B., Wellings S. R., Shyamala G., et al. Comp. Med. 2002;52:12–31. - PubMed
- Rangarajan A., Weinberg R. A. Nat. Rev. Cancer. 2003;3:952–959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous