Inhibition of cytokine gene expression and induction of chemokine genes in non-lymphatic cells infected with SARS coronavirus - PubMed (original) (raw)
Inhibition of cytokine gene expression and induction of chemokine genes in non-lymphatic cells infected with SARS coronavirus
Martin Spiegel et al. Virol J. 2006.
Abstract
Background: SARS coronavirus (SARS-CoV) is the etiologic agent of the severe acute respiratory syndrome. SARS-CoV mainly infects tissues of non-lymphatic origin, and the cytokine profile of those cells can determine the course of disease. Here, we investigated the cytokine response of two human non-lymphatic cell lines, Caco-2 and HEK 293, which are fully permissive for SARS-CoV.
Results: A comparison with established cytokine-inducing viruses revealed that SARS-CoV only weakly triggered a cytokine response. In particular, SARS-CoV did not activate significant transcription of the interferons IFN-alpha, IFN-beta, IFN-lambda1, IFN-lambda2/3, as well as of the interferon-induced antiviral genes ISG56 and MxA, the chemokine RANTES and the interleukine IL-6. Interestingly, however, SARS-CoV strongly induced the chemokines IP-10 and IL-8 in the colon carcinoma cell line Caco-2, but not in the embryonic kidney cell line 293.
Conclusion: Our data indicate that SARS-CoV suppresses the antiviral cytokine system of non-immune cells to a large extent, thus buying time for dissemination in the host. However, synthesis of IP-10 and IL-8, which are established markers for acute-stage SARS, escapes the virus-induced silencing at least in some cell types. Therefore, the progressive infiltration of immune cells into the infected lungs observed in SARS patients could be due to the production of these chemokines by the infected tissue cells.
Figures
Figure 1
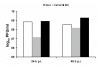
Virus titers. Simian Vero cells (white bars), human Caco-2 cells (grey bars), and human low-passage HEK 293 cells (black bars) were infected at a multiplicity of infection (MOI) of 5 infectious particles per cell. Virus titers in the supernatants were determined 24 h post-infection and 48 h post-infection by plaque assays.
Figure 2
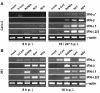
Interferon production by virus-infected human cells. Caco-2 cells (A) and HEK 293 cells (B) were infected with SARS-CoV or the IFN-inducing control viruses Bunyamwera delNSs (BdelNSs), Sendai virus (SeV), Newcastle disease virus (NDV), or were left uninfected (mock). At 8 h (left panels) or at 16 h (right panels) post-infection, total RNA was isolated and investigated by RT-PCR for the presence of different IFN mRNAs. The cellular γ-actin mRNA served as loading control. Note that for the reliable detection of IFN-α in Caco-2 cells (A, upper right panel) the infection time had to be extended to 24 h.
Figure 3
Interferon-stimulated genes. RNA samples of Caco-2 cells (A) and HEK 293 cells (B) described in Fig. 2 were investigated by RT-PCR for the presence of ISG56 and MxA mRNAs. As for IFN-α (see Fig. 2A), for detection of MxA mRNA in Caco-2 cells an extended infection period of 24 h was necessary (A, lower right panel).
Figure 4
Chemokine production. RNA samples of Caco-2 cells (A) and HEK 293 cells (B) described in Fig. 2 were assayed by RT-PCR for IP-10 and RANTES mRNA levels.
Figure 5
Interleukin production. RNA samples of Caco-2 cells (A) and HEK 293 cells (B) described in Fig. 2 were investigated by RT-PCR for the presence of IL-6 and IL-8 mRNAs.
Similar articles
- Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.
Busnadiego I, Fernbach S, Pohl MO, Karakus U, Huber M, Trkola A, Stertz S, Hale BG. Busnadiego I, et al. mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article. - Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells.
Narayanan K, Huang C, Lokugamage K, Kamitani W, Ikegami T, Tseng CT, Makino S. Narayanan K, et al. J Virol. 2008 May;82(9):4471-9. doi: 10.1128/JVI.02472-07. Epub 2008 Feb 27. J Virol. 2008. PMID: 18305050 Free PMC article. - Coordinate induction of IFN-alpha and -gamma by SARS-CoV also in the absence of virus replication.
Castilletti C, Bordi L, Lalle E, Rozera G, Poccia F, Agrati C, Abbate I, Capobianchi MR. Castilletti C, et al. Virology. 2005 Oct 10;341(1):163-9. doi: 10.1016/j.virol.2005.07.015. Virology. 2005. PMID: 16095648 Free PMC article. - Interferon and cytokine responses to SARS-coronavirus infection.
Thiel V, Weber F. Thiel V, et al. Cytokine Growth Factor Rev. 2008 Apr;19(2):121-32. doi: 10.1016/j.cytogfr.2008.01.001. Epub 2008 Mar 5. Cytokine Growth Factor Rev. 2008. PMID: 18321765 Free PMC article. Review. - Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: Case series of four critically ill patients treated with leronlimab.
Agresti N, Lalezari JP, Amodeo PP, Mody K, Mosher SF, Seethamraju H, Kelly SA, Pourhassan NZ, Sudduth CD, Bovinet C, ElSharkawi AE, Patterson BK, Stephen R, Sacha JB, Wu HL, Gross SA, Dhody K. Agresti N, et al. J Transl Autoimmun. 2021;4:100083. doi: 10.1016/j.jtauto.2021.100083. Epub 2021 Jan 6. J Transl Autoimmun. 2021. PMID: 33521616 Free PMC article. Review.
Cited by
- Inhibition of the alpha/beta interferon response by mouse hepatitis virus at multiple levels.
Roth-Cross JK, Martínez-Sobrido L, Scott EP, García-Sastre A, Weiss SR. Roth-Cross JK, et al. J Virol. 2007 Jul;81(13):7189-99. doi: 10.1128/JVI.00013-07. Epub 2007 Apr 25. J Virol. 2007. PMID: 17459917 Free PMC article. - Interferon priming enables cells to partially overturn the SARS coronavirus-induced block in innate immune activation.
Kuri T, Zhang X, Habjan M, Martínez-Sobrido L, García-Sastre A, Yuan Z, Weber F. Kuri T, et al. J Gen Virol. 2009 Nov;90(Pt 11):2686-2694. doi: 10.1099/vir.0.013599-0. Epub 2009 Jul 22. J Gen Virol. 2009. PMID: 19625461 Free PMC article. - Severe acute respiratory syndrome coronavirus elicits a weak interferon response compared to traditional interferon-inducing viruses.
Scagnolari C, Trombetti S, Cicetti S, Antonelli S, Selvaggi C, Perrone L, Visca M, Romano S, Antonelli G. Scagnolari C, et al. Intervirology. 2008;51(4):217-23. doi: 10.1159/000154258. Epub 2008 Sep 10. Intervirology. 2008. PMID: 18781076 Free PMC article. - Quantification of mRNA encoding cytokines and chemokines and assessment of ciliary function in canine tracheal epithelium during infection with canine respiratory coronavirus (CRCoV).
Priestnall SL, Mitchell JA, Brooks HW, Brownlie J, Erles K. Priestnall SL, et al. Vet Immunol Immunopathol. 2009 Jan 15;127(1-2):38-46. doi: 10.1016/j.vetimm.2008.09.017. Epub 2008 Sep 27. Vet Immunol Immunopathol. 2009. PMID: 18977539 Free PMC article.
References
- Rollins BJ. Chemokines. Blood. 1997;90:909–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous