Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge - PubMed (original) (raw)
Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge
Ana Kostic et al. Mol Biol Cell. 2006 Jun.
Abstract
Cell motility on extracellular matrices critically depends on matrix rigidity, which affects cell adhesion and formation of focal contacts. Receptor-like protein tyrosine phosphatase alpha (RPTPalpha) and the alphavbeta3 integrin form a rigidity-responsive complex at the leading edge. Here we show that the rigidity response through increased spreading and growth correlates with leading edge recruitment of Fyn, but not endogenous c-Src. Recruitment of Fyn requires the palmitoylation site near the N-terminus and addition of that site to c-Src enables it to support a rigidity response. In all cases, the rigidity response correlates with the recruitment of the Src family kinase to early adhesions. The stretch-activated substrate of Fyn and c-Src, p130Cas, is also required for a rigidity response and it is phosphorylated at the leading edge in a Fyn-dependent process. A possible mechanism for the fibronectin rigidity response involves force-dependent Fyn phosphorylation of p130Cas with rigidity-dependent displacement. With the greater displacement of Fyn from p130Cas on softer surfaces, there will be less phosphorylation. These studies emphasize the importance of force and nanometer-level movements in cell growth and function.
Figures
Figure 1.
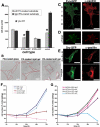
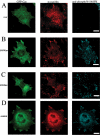
Fyn, but not Src restores rigidity response in SYF cells. (A) SYF cells spread to approximately equal area on soft and rigid FN-coated polyacrylamide gels. Reconstitution with Fyn-GFP restores rigidity response by inhibiting the spreading on soft substrates, and reconstitution with Src-GFP has no effect. Spread areas in SYF, SYF/Fyn-GFP, and SYF/Src-GFP cells were quantified, and results are presented as mean ± SE for at least 50 cells. (B) DIC images of SYF cells plated on FN-coated glass (left), rigid FN-coated polyacrylamide gels (middle), and FN-coated soft polyacrylamide gels (right). Scale bar, 20 μm. (C–F) immunofluorescence staining for the focal contact marker paxillin (red) of SYF/Fyn-GFP and SYF/Src-GFP transiently transfected cells plated on rigid (C and E) and soft FN-coated polyacrylamide gels (D and F). Fyn (green) accumulates in focal contacts that are elongated and rich in paxillin in SYF/Fyn-GFP cells plated on rigid substrates (C). On soft substrates, accumulations of Fyn and paxillin appear shorter (D). Paxillin accumulations in SYF/Src-GFP cells are shorter and punctate regardless of substrate rigidity (E and F). Scale bar, 20 μm. (G) The proliferation on rigid versus soft FN-coated gels was observed over 72 h upon plating. The number of cells was counted and normalized by the number of cells initially plated. RPTPa+/+ cells were used as control. Although control cells proliferate much faster on rigid than on soft substrates, SYF cells grew equally well regardless of substrate rigidity. Introduction of Fyn-GFP, but not Src-GFP rescued wild-type phenotype on soft substrates.
Figure 2.
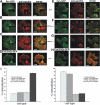
Localization of Fyn to early focal contacts is RPTPα-dependent. (A–C) immunofluorescence staining for paxillin (red) of SYF/Src-GFP (A), RPTPα+/+/Src-GFP (B), and RPTPα−/−/Src-GFP (C) fibroblast cells plated on FN-coated glass for 30 min show low concentration of Src-GFP in early focal contacts. (D) Immunostaining of SYF/Src(S3C)-GFP shows increased recruitment of Src palmitoylated mutant to focal contacts. (E and F) Immunofluorescence stainings for paxillin (red) of SYF/Fyn-GFP (E) and RPTPα+/+/Fyn-GFP (F) cells show colocalization of Fyn-GFP and paxillin in early focal contacts. (G and H) Immunofluorescence stainings for paxillin (red) of RPTPα−/−/Src-GFP (G) and SYF/Fyn(C3G)-GFP (H) cells show reduced overlap in localization of Fyn and paxillin due to the lack of RPTPα-mediated activation of Fyn and palmitoylation, respectively. Scale bar, 10 μm. Blow-up panels are 2.5× magnified sections marked by a dash line. (I) Quantitative analysis of colocalization of GFP-fusion proteins with paxillin staining. Fluorescence intensities were measured across focal contacts (identified as areas with increased red signal toward the edge of the cells) for both channels; background fluorescence was subtracted, and the ratio between intensities in green (GFP-fusion proteins) and red channel (paxillin) was calculated. Cells included in analysis were representative of the larger cell population; at least 10 cells with a minimum of 35 focal contacts were analyzed for each condition.
Figure 3.
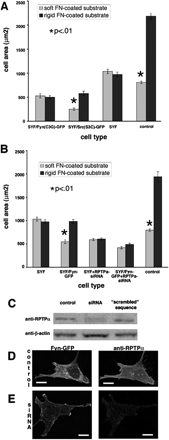
Fyn-mediated rigidity response requires both palmitoylation and RPTPα. (A) Quantitation of spread areas of SYF/Fyn(C3G)-GFP and SYF/Src(S3C)-GFP cells showing that the loss of the palmitoylation site in the N-terminus of Fyn kinase blocks its ability to restore rigidity response, whereas introduction of the palmitoylation site in the N-terminus of Src kinase enabled it to restore rigidity response in SYF cells. Results shown as mean ± SE for at least 50 cells (same as in B). (B) Quantitation of spread areas of SYF/Fyn-GFP cells treated with siRNA for RPTPα. Despite the presence of Fyn-GFP, no significant difference in spread area was observed between cells on rigid versus soft FN-coated polyacrylamide substrates, indicating that RPTPα activation of Fyn is necessary for rigidity response. (C) Western blots showing decreased levels of RPTPα in RPTPα-siRNA–treated cells compared with untreated control and control treated with “scrambled” sequence. Loading control is β-actin. (D and E) Immunofluorescence staining for RPTPα in control cells (D) and RPTPα-siRNA-treated SYF/Fyn-GFP cells (E) shows decrease in RPTPα expression levels in treated cells. Scale bar, 15 μm.
Figure 4.
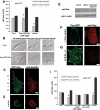
Activation of Cas is required in Fyn-mediated FN rigidity response. (A) Cas−/− cells spread to approximately equal area on soft and rigid FN-coated polyacrylamide gels (similar to SYF cell; see Figure 1). Reconstitution with GFP-Cas restored the rigidity response by increasing spreading on rigid substrates. Results are presented as the mean spread area ± SE for at least 50 cells. (B) DIC images of Cas−/− (top panel) and Cas−/−/GFP-Cas cells (bottom panel) plated on FN-coated glass (left), rigid FN-coated polyacrylamide gels (middle), and soft FN-coated polyacrylamide gels (right). Scale bars, 20 μm. (C and D) Immunofluorescence staining for the focal contact marker paxillin (red) of Cas−/−/GFP-Cas transiently transfected cells plated on rigid (C) and soft FN-coated polyacrylamide gels (D). GFP-Cas (green) accumulated in focal contacts that were elongated and rich in paxillin in cells plated on rigid substrates (C). On soft substrates, accumulations of GFP-Cas were shorter and punctate (D). Scale bar, 10 μm. (E) Western blots showing decreased levels of Cas in Cas-siRNA–treated cells compared with untreated control and control treated with “scrambled” sequence. Loading control is β-actin. (F and G) Immunofluorescence staining for Cas (red) in control cells (G) and Cas-siRNA–treated SYF/Fyn-GFP cells (I) shows decrease in Cas expression levels in treated cells. Scale bar, 10 μm. (I) Quantitation of spread area of SYF/Fyn-GFP cells treated with siRNA for Cas. Results are shown as mean ± SE for at least 50 cells. Despite the presence of Fyn-GFP, no significant difference in spread area was observed between cells on rigid versus soft FN-coated polyacrylamide substrates, indicating that Cas phosphorylation by Fyn was necessary for the rigidity response.
Figure 5.
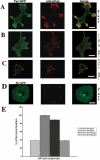
Fyn, unlike Src rescues recruitment of Cas to early focal contacts in SYF cells. (A) Transiently transfected SYF/GFP-Cas cells were plated on FN-coated glass for 45 min, fixed, and immunostained for paxillin (red). The amount of GFP-Cas (green) in focal contacts labeled with anti-paxillin antibody (red) was much lower than in control cells (D). Reconstitution with Fyn significantly increased the amount of GFP-Cas in focal contacts (B), whereas reconstitution with Src had no effect (C). The activity of Fyn/Src was quantified by immunofluorescent staining with an anti-phosphoY416-Src family kinase antibody that recognizes the activated form of SFKs (cyan). Scale bar, 10 μm.
Figure 6.
Fyn-mediated phosphorylation of Cas depends on matrix rigidity. (A–C) SYF/Fyn-GFP cells were plated for 1 h on FN-coated glass (A), rigid gel (B), and soft gel (C). Left panels, distribution of Fyn-GFP; middle panels, immunostainings for pY165Cas (phosphorylated Cas); and right panels, merge. (D) Immunostaining of SYF/Src-GFP cells plated for 1 h on FN-coated glass for pY165Cas (phosphorylated Cas). Scale bar, 10 μm. (E) Quantitative analysis of immunostainings for pY165Cas (phosphorylated Cas). Fluorescence intensities were measured across focal contacts (identified as areas with increased red signal toward the edge of the cells) for red channel; background fluorescence was subtracted, and average intensity was calculated. Cells included in analysis were representative of the larger cell population; at least 10 cells with minimum of 35 focal contacts were analyzed for each condition. Results are normalized against the average pCas signal in SYF/Fyn-GFP cells plated on FN-coated glass.
Figure 7.
Model of rigidity response though RPTPα-mediated Fyn activation. Schematic drawing depicts how RPTPα-mediated activation of Fyn upon cell adhesion to FN-coated substrate regulates rigidity response. Cells attach to rigid or soft FN-coated substrate through αvβ3 integrins, which causes formation of the integrin–cytoskeleton complex at the leading edge. This complex is connected to acto-myosin gel via actin-binding proteins in the complex. Inactive Fyn (pink) is bound to membrane due to palmitoylation. Force is exerted on the leading edge complex in response to matrix rigidity. At low forces, RPTPα is activated by a conformation change in the αvβ3 integrin, and it subsequently activates Fyn by dephosphorylating it. Simultaneously, rearward movement of actin begins driven by myosin motors. The leading edge complex moves along with the actin network, but at a slower rate because of the slip bond between integrins and cytoskeleton. On rigid substrates force is developed rapidly; therefore, it reaches the critical force (Fc) required for Fyn or Cas activation while all components are in close proximity. On soft substrates, the displacement needed to reach the activation force threshold is greater; therefore, inactive Cas (blue) moves away from Fyn before force-dependent activation can cause phosphorylation. On rigid substrates, phosphorylation of Cas (red) is followed by accumulation of focal contact proteins and reinforcement of the integrin–cytoskeleton bond. This stabilizes leading edge and accelerates cell spreading. On soft substrates, unphosphorylated Cas fails to play its role in reinforcement, which results in impaired spreading.
Similar articles
- RPTPalpha is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons.
Kostic A, Sap J, Sheetz MP. Kostic A, et al. J Cell Sci. 2007 Nov 1;120(Pt 21):3895-904. doi: 10.1242/jcs.009852. Epub 2007 Oct 16. J Cell Sci. 2007. PMID: 17940065 - RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages.
von Wichert G, Jiang G, Kostic A, De Vos K, Sap J, Sheetz MP. von Wichert G, et al. J Cell Biol. 2003 Apr 14;161(1):143-53. doi: 10.1083/jcb.200211061. Epub 2003 Apr 7. J Cell Biol. 2003. PMID: 12682088 Free PMC article. - Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts.
Su J, Muranjan M, Sap J. Su J, et al. Curr Biol. 1999 May 20;9(10):505-11. doi: 10.1016/s0960-9822(99)80234-6. Curr Biol. 1999. PMID: 10339427 - Cyclic stretch induces reorientation of cells in a Src family kinase- and p130Cas-dependent manner.
Niediek V, Born S, Hampe N, Kirchgessner N, Merkel R, Hoffmann B. Niediek V, et al. Eur J Cell Biol. 2012 Feb;91(2):118-28. doi: 10.1016/j.ejcb.2011.10.003. Epub 2011 Dec 16. Eur J Cell Biol. 2012. PMID: 22178114 - Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha.
Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Jiang G, et al. Biophys J. 2006 Mar 1;90(5):1804-9. doi: 10.1529/biophysj.105.072462. Epub 2005 Dec 9. Biophys J. 2006. PMID: 16339875 Free PMC article.
Cited by
- Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression.
Schedin P, Keely PJ. Schedin P, et al. Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a003228. doi: 10.1101/cshperspect.a003228. Cold Spring Harb Perspect Biol. 2011. PMID: 20980442 Free PMC article. Review. - Early events in cell spreading as a model for quantitative analysis of biomechanical events.
Wolfenson H, Iskratsch T, Sheetz MP. Wolfenson H, et al. Biophys J. 2014 Dec 2;107(11):2508-14. doi: 10.1016/j.bpj.2014.10.041. Epub 2014 Dec 2. Biophys J. 2014. PMID: 25468330 Free PMC article. Review. - Growth control by intracellular tension and extracellular stiffness.
Assoian RK, Klein EA. Assoian RK, et al. Trends Cell Biol. 2008 Jul;18(7):347-52. doi: 10.1016/j.tcb.2008.05.002. Epub 2008 May 29. Trends Cell Biol. 2008. PMID: 18514521 Free PMC article. Review. - CAS proteins in normal and pathological cell growth control.
Tikhmyanova N, Little JL, Golemis EA. Tikhmyanova N, et al. Cell Mol Life Sci. 2010 Apr;67(7):1025-48. doi: 10.1007/s00018-009-0213-1. Epub 2009 Nov 25. Cell Mol Life Sci. 2010. PMID: 19937461 Free PMC article. Review. - Transglutaminase-mediated oligomerization promotes osteoblast adhesive properties of osteopontin and bone sialoprotein.
Forsprecher J, Wang Z, Goldberg HA, Kaartinen MT. Forsprecher J, et al. Cell Adh Migr. 2011 Jan-Feb;5(1):65-72. doi: 10.4161/cam.5.1.13369. Epub 2011 Jan 1. Cell Adh Migr. 2011. PMID: 20864802 Free PMC article.
References
- Abassi Y. A., Rehn M., Ekman N., Alitalo K., Vuori K. p130Cas Couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskeleton and cell migration. J. Biol. Chem. 2003;278:35636–35643. - PubMed
- Alland L., Peseckis S. M., Atherton R. E., Berthiaume L., Resh M. D. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 1994;269:16701–16705. - PubMed
- Anders D. L., Blevins T., Sutton G., Chandler L. J., Woodward J. J. Effects of c-Src tyrosine kinase on ethanol sensitivity of recombinant NMDA receptors expressed in HEK 293 cells. Alcohol Clin. Exp. Res. 1999a;23:357–362. - PubMed
- Anders D. L., Blevins T., Sutton G., Swope S., Chandler L. J., Woodward J. J. Fyn tyrosine kinase reduces the ethanol inhibition of recombinant NR1/NR2A but not NR1/NR2B NMDA receptors expressed in HEK 293 cells. J. Neurochem. 1999b;72:1389–1393. - PubMed
- Ardini E., Agresti R., Tagliabue E., Greco M., Aiello P., Yang L. T., Menard S., Sap J. Expression of protein tyrosine phosphatase alpha (RPTPalpha) in human breast cancer correlates with low tumor grade, and inhibits tumor cell growth in vitro and in vivo. Oncogene. 2000;19:4979–4987. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous