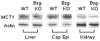Tissue distribution of basigin and monocarboxylate transporter 1 in the adult male mouse: a study using the wild-type and basigin gene knockout mice - PubMed (original) (raw)
Tissue distribution of basigin and monocarboxylate transporter 1 in the adult male mouse: a study using the wild-type and basigin gene knockout mice
Masaaki Nakai et al. Anat Rec A Discov Mol Cell Evol Biol. 2006 May.
Abstract
Basigin (Bsg) is a transmembrane protein that is responsible for targeting of monocarboxylate transporters (MCTs) to the cell membrane. The present study was conducted to determine whether or not Bsg was required for the proper localization of MCT isoform 1 (MCT1) in a wide range of tissues in adult male mice. The tissue distributions of Bsg and MCT1 in wild-type (WT) mice, the tissue distribution of MCT1 in Bsg gene knockout (Bsg-KO) mice, and the protein and mRNA levels of MCT1 in both genotypes were studied. Immunohistochemistry demonstrated that Bsg colocalized with MCT1 in the cerebrum, retina, skeletal and cardiac muscle, duodenal epithelium, hepatic sinusoid, proximal uriniferous tubules, Leydig cells, and efferent ductule epithelium in WT mice. Bsg was absent but MCT1 was present in Sertoli cells, cauda epididymis, myoepithelial cells and duct of the mandibular gland, surface epithelium of the stomach and bronchioles. In Bsg-KO mice, with the exception of Leydig cells, MCT1 immunostaining was greatly reduced in intensity and its distribution was altered in tissues that expressed both Bsg and MCT1 in WT mice. Levels of the protein and mRNA for MCT1 in these tissues did not change significantly in Bsg-KO mice. On the other hand, immunostaining patterns in cells in which Bsg was absent but MCT1 was present in WT mice remained unchanged in Bsg-KO mice. These observations suggest that Bsg is required for the proper localization of MCT1 in a wide range of cells but not in every cell type.
2006 Wiley-Liss, Inc.
Figures
Figure 1
Western blotting analyses for Bsg and MCT1 proteins. a: Lung proteins of the WT and Bsg-KO mice probed for Bsg. A broad band characteristic of Bsg is seen in the WT but no band is seen in the Bsg-KO, confirming the specificity of the antibody. b: Lung proteins of the WT mouse probed with an anti-MCT1 antibody and pre-immune normal chicken IgY. The antibody produces a band at 43 kDa, which is not seen when probed with normal IgY. c: Basigin is present in all organs studied in the WT. The molecular weight (MW) ranges from approximately 26 to 65 kDa, with the largest MW in the eye, kidney (Ki) and stomach (St) and the lowest MW in the caput epididymis (CpE) and cauda epididymis (CdE). Sk: skeletal muscle, Ce: cerebrum, Te: testis, Sv: seminal vesicle, Du: duodenum, Li: liver, He: heart, Lu: lung, Sa: salivary gland (mandibular and sublingual glands).
Figure 2
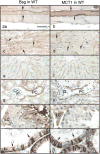
Immunohistochemistry for Bsg (left column) and MCT1 (right column) in Group 1 of the WT mice. Bar = 50 μm. In skeletal muscle fibers, Bsg is seen on the cell surface (a, arrows) and MCT1 is in the cytoplasm and on the cell surface (b, arrows). In cardiac muscle fibers, Bsg (c) and MCT1 (d) are seen on the cell surface, especially at the intercalated discs (arrows). In the liver, Bsg (e) and MCT1 (f) are seen along the sinusoidal wall. In the kidney, Bsg (g) and MCT1 (h) are seen in the basal cytoplasm of epithelial cells lining the initial part of the proximal convoluted tubule (PC). G: glomerulus. In the testis, Bsg (i) is present in the sperm tail (arrow) and in the Leydig cell (LC) surface. MCT1 (j) is seen in Leydig cells (LC). In the efferent ductules, intense Bsg (k) and MCT1 (l) immunoreactions are seen on the basolateral surface of ciliated cells (arrows).
Figure 3
Immunohistochemistry for Bsg (left column) and MCT1 (right column) in Group 2 of the WT mice. Bar = 50 μm. In the cauda epididymis, Bsg (a) is negative except for sperm tails but MCT1 is strongly positive along the brush border of the epithelium (b). In the mandibular gland, Bsg (c) is absent but MCT1 is present on the duct epithelium (arrow) and myoepithelial cells (arrowheads). In the stomach, Bsg is absent (e, arrows) but MCT1 is present in gastric superficial cells (f, arrows). Note that parietal cells (arrowhead) are positive for Bsg on the basolateral surface but negative for MCT1.
Figure 4
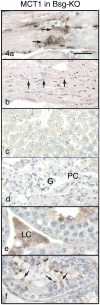
Immunohistochemistry of MCT1 in Group 1 of the Bsg-KO mice. Bar = 50 μm. In the skeletal (a) and cardiac (b) muscles, MCT1 disappears from the cell surface and cytoplasm but accumulates in the perinuclear area (arrows). No MCT1 immunostaining is seen in the liver (c) and kidney (d). G: gromerulus, PC: Proximal convoluted tubule. In the testis, Leydig cells (e, LC) remain positive for MCT1 in the absence of Bsg. In the efferent ductules (f), MCT1 disappears from the basolateral surface and accumulates on the apical border of ciliated cells (arrows).
Figure 5
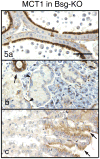
Immunohistochemistry of MCT1 in Group 2 of the Bsg-KO mice. Bar = 50 μm. Immunostaining patterns of MCT1 in the cauda epididymis (a), mandibular gland (b) and stomach (c) in the Bsg-KO mice do not differ significantly from those in the WT.
Figure 6
Western blotting analysis of MCT1 protein levels in the liver, caput epididymis (Cap Epi) and kidney of WT and Bsg-KO mice. There is no significant difference in MCT1 protein level in any organ between the two genotypes. Actin is used as an internal loading control.
Similar articles
- Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse.
Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Philp NJ, et al. Invest Ophthalmol Vis Sci. 2003 Mar;44(3):1305-11. doi: 10.1167/iovs.02-0552. Invest Ophthalmol Vis Sci. 2003. PMID: 12601063 - Characterization of the Expression of Basigin Gene Products Within the Pineal Gland of Mice.
Tokar D, van Ekeris L, Linser PJ, Ochrietor JD. Tokar D, et al. Cell Mol Neurobiol. 2017 Aug;37(6):1141-1145. doi: 10.1007/s10571-016-0441-5. Epub 2016 Nov 4. Cell Mol Neurobiol. 2017. PMID: 27815658 Free PMC article. - Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells.
Philp NJ, Wang D, Yoon H, Hjelmeland LM. Philp NJ, et al. Invest Ophthalmol Vis Sci. 2003 Apr;44(4):1716-21. doi: 10.1167/iovs.02-0287. Invest Ophthalmol Vis Sci. 2003. PMID: 12657613 - Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion.
Muramatsu T, Miyauchi T. Muramatsu T, et al. Histol Histopathol. 2003 Jul;18(3):981-7. doi: 10.14670/HH-18.981. Histol Histopathol. 2003. PMID: 12792908 Review. - The SLC16 gene family - structure, role and regulation in health and disease.
Halestrap AP. Halestrap AP. Mol Aspects Med. 2013 Apr-Jun;34(2-3):337-49. doi: 10.1016/j.mam.2012.05.003. Mol Aspects Med. 2013. PMID: 23506875 Review.
Cited by
- Expression of basigin in reproductive tissues of estrogen receptor-{alpha} or -{beta} null mice.
Chen L, Bi J, Nakai M, Bunick D, Couse JF, Korach KS, Nowak RA. Chen L, et al. Reproduction. 2010 Jun;139(6):1057-66. doi: 10.1530/REP-10-0069. Epub 2010 Apr 13. Reproduction. 2010. PMID: 20388736 Free PMC article. - Comparisons of mass spectrometry compatible surfactants for global analysis of the mammalian brain proteome.
Chen EI, McClatchy D, Park SK, Yates JR 3rd. Chen EI, et al. Anal Chem. 2008 Nov 15;80(22):8694-701. doi: 10.1021/ac800606w. Epub 2008 Oct 21. Anal Chem. 2008. PMID: 18937422 Free PMC article. - Validation of oligoarrays for quantitative exploration of the transcriptome.
Nygaard V, Liu F, Holden M, Kuo WP, Trimarchi J, Ohno-Machado L, Cepko CL, Frigessi A, Glad IK, Wiel MA, Hovig E, Lyng H. Nygaard V, et al. BMC Genomics. 2008 May 30;9:258. doi: 10.1186/1471-2164-9-258. BMC Genomics. 2008. PMID: 18513391 Free PMC article. - pH and male fertility: making sense on pH homeodynamics throughout the male reproductive tract.
Bernardino RL, Carrageta DF, Sousa M, Alves MG, Oliveira PF. Bernardino RL, et al. Cell Mol Life Sci. 2019 Oct;76(19):3783-3800. doi: 10.1007/s00018-019-03170-w. Epub 2019 Jun 4. Cell Mol Life Sci. 2019. PMID: 31165202 Free PMC article. Review. - Loss of basigin expression in uterine cells leads to subfertility in female mice†.
Li K, Li Q, Bashir ST, Bany BM, Nowak RA. Li K, et al. Biol Reprod. 2021 Oct 11;105(4):859-875. doi: 10.1093/biolre/ioab109. Biol Reprod. 2021. PMID: 34106247 Free PMC article.
References
- Betsuyaku T, Kadomatsu K, Griffin GL, Muramatsu T, Senior RM. Increased basigin in bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2003;28:600–606. - PubMed
- Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. - PubMed
- Bonen A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur J Appl Physiol. 2001;86:6–11. - PubMed
- Cesario MM, Bartles JR. Compartmentalization, processing and redistribution of the plasma membrane protein CE9 on rodent spermatozoa. Relationship of the annulus to domain boundaries in the plasma membrane of the tail. J Cell Sci. 1994;107:561–570. - PubMed
- Cesario MM, Ensrud K, Hamilton DW, Bartles JR. Biogenesis of the posterior-tail plasma membrane domain of the mammalian spermatozoon: targeting and lateral redistribution of the posterior-tail domain-specific transmembrane protein CE9 during spermiogenesis. Dev Biol. 1995;169:473–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous