Basic fibroblast growth factor protects against rotenone-induced dopaminergic cell death through activation of extracellular signal-regulated kinases 1/2 and phosphatidylinositol-3 kinase pathways - PubMed (original) (raw)
Basic fibroblast growth factor protects against rotenone-induced dopaminergic cell death through activation of extracellular signal-regulated kinases 1/2 and phosphatidylinositol-3 kinase pathways
Shih-Ling Hsuan et al. J Neurosci. 2006.
Abstract
Administration of rotenone to rats reproduces many features of Parkinson's disease, including dopaminergic neuron degeneration, and provides a useful model to study the pathogenesis of Parkinson's disease. However, the cell death mechanisms induced by rotenone and potential neuroprotective mechanisms against rotenone are not well defined. Here we report that rotenone-induced apoptosis in human dopaminergic SH-SY5Y cells is attenuated by pretreatment with several growth factors, most notably basic fibroblast growth factor (bFGF). bFGF activated both extracellular signal-regulated kinase 1/2 (ERK1/2) and phosphatidylinositol-3 kinase (PI3-kinase) pathways in SH-SY5Y cells. Ectopic activation of ERK1/2 or PI3-kinase protected against rotenone, whereas inhibition of either pathway attenuated bFGF protection. Reducing the expression of the proapoptotic protein Bcl-2-associated death protein (BAD) by small interfering RNA rendered SH-SY5Y cells resistant to rotenone, implicating BAD in rotenone-induced cell death. Interestingly, bFGF induced a long-lasting phosphorylation of BAD at serine 112, suggesting BAD inactivation through the ERK1/2 signaling pathway. Moreover, primary cultured dopaminergic neurons from mesencephalon were more sensitive to rotenone-induced cell death than nondopaminergic neurons in the same culture. The loss of dopaminergic neurons was blocked by bFGF, an inhibition dependent on ERK1/2 and PI3-kinase signaling. These data suggest that rotenone-induced dopaminergic cell death requires BAD and identify bFGF and its activation of ERK1/2 and PI3-kinase signaling pathways as novel intervention strategies to block cell death in the rotenone model of Parkinson's disease.
Figures
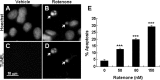
Figure 1.
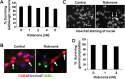
Rotenone induces apoptosis in SH-SY5Y cells. A–D, Representative photomicrographs of nuclear morphology (A, B) and TUNEL staining (C, D) of SH-SY5Y cells treated with vehicle control (A, C) or 50 n
m
rotenone for 24 h (B, D). Nuclear morphology was visualized after Hoechst staining. Arrows identify cells with condensed or fragmented nuclei, characteristic of apoptosis. Scale bar, 10 μm. E, Dose–response of rotenone-induced apoptosis. SH-SY5Y cells were treated with vehicle, 50, 80, or 150 n
m
rotenone for 24 h. Apoptosis was scored based on nuclear morphology after Hoechst staining. Data are from four independent experiments of duplicate determinations. At least 8000 cells were counted for each data point. Error bars represent SEM. ***p < 0.001 (ANOVA) compared with control.
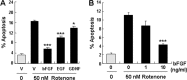
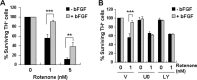
Figure 2.
Effect of growth factors on rotenone-induced SH-SY5Y cell apoptosis. A, SH-SY5Y cells were preincubated with 10 ng/ml bFGF, EGF, or GDNF for 1 h before treatment with 50 n
m
rotenone for 24 h. B, Dose–response of bFGF protection. Cells were preincubated with 0–10 ng/ml bFGF for 1 h, followed by 50 n
m
rotenone treatment for 24 h. Data are from two independent experiments of triplicate determinations. At least 6000 cells were counted for each data point. Error bars are SEM. *p < 0.05, ***p < 0.001 (ANOVA) compared with cells treated with rotenone only. V, Vehicle.
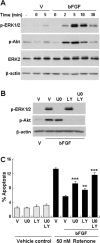
Figure 3.
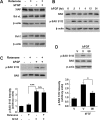
bFGF protects SH-SY5Y cells against rotenone via activation of ERK1/2 and PI3-kinase. A, Kinetics of ERK1/2 and PI3-kinase activation by 10 ng/ml bFGF. B, SH-SY5Y cells were pretreated with vehicle control DMSO (V), 10 μ
m
U0126 (U0), or 10 μ
m
LY294002 (LY) for 1 h before bFGF stimulation (10 ng/ml, 5 min). Cell lysates were harvested and subjected to Western blot analysis for phospho-ERK1/2 (p-ERK1/2) or phospho-Akt (p-Akt). β-Actin and total ERK2 were used as loading controls. Immunoblots were representative of three independent experiments. C, Inhibition of ERK1/2 or PI3-kinase pathways reverses bFGF protection against rotenone. SH-SY5Y cells were pretreated with vehicle control, 10 μ
m
U0126, or 10 μ
m
LY294002 for 1 h, followed by 10 ng/ml bFGF for another hour before incubation with rotenone (50 n
m
, 24 h). Data are from two independent experiments of triplicate determinations. At least 6000 cells were counted for each data point. Error bars are SEM. **p < 0.01, ***p < 0.001 (ANOVA) compared with cells cotreated with rotenone and bFGF.
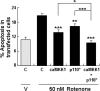
Figure 4.
Activation of ERK1/2 or PI3-kinase signaling pathway is sufficient to protect SH-SY5Y cells against rotenone-induced apoptosis. SH-SY5Y cells were transfected with 1 μg of plasmid DNA encoding caMKK1, p110*, or both. All cells were cotransfected with 0.5 μg of plasmid DNA encoding eGFP as a marker to identify transfected cells. Vector plasmid DNA was used as a control (C) and to supplement total amount of plasmid DNA in each 35 mm plate to 2.5 μg. Two days after transfection, cells were treated with 50 n
m
rotenone or vehicle control (V) for 24 h, and apoptosis in transfected cells (eGFP+) was analyzed. Data are from two independent experiments with triplicate determinations. At least 1200 transfected cells were counted for each data point. Error bars are SEM. *p < 0.05, **p < 0.01, ***p < 0.001 (ANOVA) compared with control-transfected, rotenone-treated group.
Figure 5.
bFGF activation of ERK1/2 signaling induces BAD phosphorylation at serine 112 in SH-SY5Y cells. A, Rotenone or bFGF does not change protein expression of XIAP, Bcl-xL, or Bcl-2. SH-SY5Y cells were treated with rotenone (50 n
m
), bFGF (10 ng/ml), or the two together for 24 h, and cell lysates were analyzed for XIAP, Bcl-xL, or Bcl-2 expression by Western blotting. Immunoblots were reprobed with an anti-β-actin antibody as a loading control. B, bFGF does not change BAD protein expression but induces a prolonged phosphorylation of BAD at Ser112. SH-SY5Y cells were stimulated with 10 ng/ml bFGF for 0–24 h, and cell lysates were analyzed by immunoblotting with antibodies recognizing total BAD or BAD phosphorylated at Ser112 (p-BAD S112). C, bFGF induces BAD phosphorylation at Ser112 even in the presence of rotenone. Cells were treated with bFGF and rotenone as in A. D, bFGF phosphorylation of BAD at serine 112 is mediated via the ERK1/2 pathway. Cells were pretreated for 1 h with 10 μ
m
U0126 (U0) or vehicle control (V), followed by 1 h treatment with 10 ng/ml bFGF. Immunoblots are representative of at least three independent experiments. Relative BAD phosphorylation in C and D were obtained from four to five Western blots and normalized to total BAD protein levels. Error bars are SEM. *p < 0.05, ***p < 0.001 (ANOVA). ns, Not statistically significant.
Figure 6.
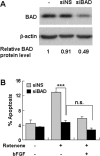
siRNA knockdown of BAD expression abolishes rotenone-induced apoptosis in SH-SY5Y cells. A, Transfection of siBAD, but not siNS, reduces BAD protein expression. SH-SY5Y cells were transfected with a 100 n
m
concentration of an siRNA specific to human BAD or a FITC-labeled doubled-stranded RNA oligomer control. BAD protein expression was analyzed 2 d later by Western blotting. The relative expression levels of BAD were normalized to the loading control β-actin. B, Reduced BAD expression renders SH-SY5Y cells resistant to rotenone. Cells were transfected with siRNA as in A. Cells were also cotransfected with plasmid DNA encoding eGFP to label transfected cells. Two days after transfection, cells were pretreated with 10 ng/ml bFGF or vehicle control for 1 h, followed by 50 n
m
rotenone or vehicle control for 24 h. Apoptosis were scored in eGFP-positive cell population. Data are representative of three independent experiments. At least 600 transfected cells were counted in each data point. Error bars are SEM. ***p < 0.001; ns, not significant (ANOVA).
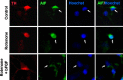
Figure 7.
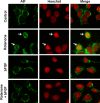
bFGF blocks rotenone-induced AIF nuclear translocation in SH-SY5Y cells. SH-SY5Y cells were pretreated with 10 ng/ml bFGF or vehicle control for 1 h, followed by 0 or 100 n
m
rotenone for 12 h. Cells were stained with an anti-AIF antibody and Hoechst. Data are deconvolution confocal images and representative of two independent experiments with triplicate determinations. Hoechst staining was pseudocolored to red to better visualize colocalization with AIF. Arrows point to cells with nuclear AIF staining.
Figure 8.
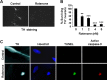
Rotenone induces dopaminergic neuron apoptosis in E14 ventral mesencephalic primary cultures. Primary ventral mesencephalic cultures were prepared from E14 rats and treated with rotenone for 24 h. A, Representative photomicrographs of cells treated with 5 n
m
rotenone or vehicle control for 24 h and immunostained for TH, a marker for dopaminergic neurons. B, Rotenone induces a dose-dependent cell loss of TH+ neurons. All TH+ cells on each coverslip were counted. Vehicle control represents 100% survival, which has ∼100 TH+ neurons per coverslip. C, Representative deconvolution confocal photomicrographs of cell treated with 2 n
m
rotenone or vehicle control for 18 h. Cells were TUNEL labeled, followed by immunostaining for active caspase-3 and TH. Data are representative of at least two independent experiments with duplicate determinations. Error bars are SEM. **p < 0.01, ***p < 0.001 (ANOVA) compared with vehicle control-treated group.
Figure 9.
Rotenone does not induce cell loss of GABAergic neurons or the general population. Primary ventral mesencephalic cultures were prepared from E14 rats and treated with 0–5 n
m
rotenone for 24 h. A, Cells were immunostained for GABA, and the number of GABA+ cells in 10 randomly selected fields was counted. Vehicle control represents 100% survival. B, Representative photomicrographs of cells treated with rotenone or vehicle control for 24 h and stained for TUNEL (green), GABA (red), and Hoechst (blue). Arrows point to GABA+ neurons that are negative for TUNEL. C, Representative photomicrographs of nuclear morphology of the entire population, revealed by Hoechst staining. Cells were treated with rotenone as in A. Arrows point to condensed or fragmented nuclei, characteristic of apoptosis. D, Rotenone treatment did not affect survival of the general population based on nuclear morphological changes revealed by Hoechst staining as in C. Data are representative of two (A) or three (D) independent experiments with duplicate determinations. At least 2000 cells were counted for each condition.
Figure 10.
bFGF blocks rotenone-induced AIF nuclear translocation in primary cultured dopaminergic cells. Primary ventral mesencephalic cultures were pretreated with 10 ng/ml bFGF for 1 h before 2 n
m
rotenone treatment for 12 h when indicated. Images are representative deconvolution confocal photomicrographs of cells stained for TH (red), AIF (green), and Hoechst (blue). Arrows point to the nuclei of TH+ neurons. Data are representatives of two independent experiments with triplicate determinations.
Figure 11.
bFGF protects primary dopaminergic neurons against rotenone-induced cell death via activation of ERK1/2 and PI3-kinase pathways. A, bFGF reduces rotenone-induced loss of TH+ dopaminergic neurons. Primary ventral mesencephalic cultures were pretreated with 10 ng/ml bFGF for 1 h, followed by 1–5 n
m
rotenone treatment for 24 h. Data were from two independent experiments with duplicate determinations. B, bFGF protection against rotenone is reversed by pharmacological inhibition of ERK1/2 or PI3-kinase pathways. Primary ventral mesencephalic cultures were pretreated with bFGF for 1 h, followed by a 24 h treatment with rotenone, 10 μ
m
U0126 (U0), 10 μ
m
LY294002 (LY), or vehicle control (V) as indicated. All TH+ cells on each coverslip were counted. Vehicle control represents 100% survival. Data are representative of two independent experiments with triplicate determinations. Error bars are SEM. **p < 0.01, ***p < 0.001 (ANOVA).
Similar articles
- Activation of c-Jun N-terminal protein kinase is a common mechanism underlying paraquat- and rotenone-induced dopaminergic cell apoptosis.
Klintworth H, Newhouse K, Li T, Choi WS, Faigle R, Xia Z. Klintworth H, et al. Toxicol Sci. 2007 May;97(1):149-62. doi: 10.1093/toxsci/kfm029. Epub 2007 Feb 25. Toxicol Sci. 2007. PMID: 17324951 - Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage.
Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J. Zhu Y, et al. J Neurosci. 2002 May 15;22(10):3898-909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. J Neurosci. 2002. PMID: 12019309 Free PMC article. - ERK1/2 antagonizes glycogen synthase kinase-3beta-induced apoptosis in cortical neurons.
Hetman M, Hsuan SL, Habas A, Higgins MJ, Xia Z. Hetman M, et al. J Biol Chem. 2002 Dec 20;277(51):49577-84. doi: 10.1074/jbc.M111227200. Epub 2002 Oct 21. J Biol Chem. 2002. PMID: 12393899 - Differential effect of nerve growth factor on dopaminergic neurotoxin-induced apoptosis.
Hirata Y, Meguro T, Kiuchi K. Hirata Y, et al. J Neurochem. 2006 Oct;99(2):416-25. doi: 10.1111/j.1471-4159.2006.04006.x. J Neurochem. 2006. PMID: 17029596 - Signaling pathways mediating anti-apoptotic action of neurotrophins.
Hetman M, Xia Z. Hetman M, et al. Acta Neurobiol Exp (Wars). 2000;60(4):531-45. doi: 10.55782/ane-2000-1374. Acta Neurobiol Exp (Wars). 2000. PMID: 11200182 Review.
Cited by
- Changes in the vitreous body after experimental vitreous hemorrhage in rabbit: An interdisciplinary study.
Zhang P, Yan W, Yan H. Zhang P, et al. PLoS One. 2023 Feb 6;18(2):e0281165. doi: 10.1371/journal.pone.0281165. eCollection 2023. PLoS One. 2023. PMID: 36745670 Free PMC article. - Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain.
Chen Z, Jalabi W, Hu W, Park HJ, Gale JT, Kidd GJ, Bernatowicz R, Gossman ZC, Chen JT, Dutta R, Trapp BD. Chen Z, et al. Nat Commun. 2014 Jul 22;5:4486. doi: 10.1038/ncomms5486. Nat Commun. 2014. PMID: 25047355 Free PMC article. - Expression and immunolocalization of the plasma membrane monoamine transporter in the brain.
Dahlin A, Xia L, Kong W, Hevner R, Wang J. Dahlin A, et al. Neuroscience. 2007 May 25;146(3):1193-211. doi: 10.1016/j.neuroscience.2007.01.072. Epub 2007 Apr 3. Neuroscience. 2007. PMID: 17408864 Free PMC article. - Effect of moxibustion on mTOR-mediated autophagy in rotenone-induced Parkinson's disease model rats.
Wang SJ, Wang Q, Ma J, Yu PH, Wang ZM, Wang B. Wang SJ, et al. Neural Regen Res. 2018 Jan;13(1):112-118. doi: 10.4103/1673-5374.224380. Neural Regen Res. 2018. PMID: 29451215 Free PMC article. - Rotenone and paraquat do not directly activate microglia or induce inflammatory cytokine release.
Klintworth H, Garden G, Xia Z. Klintworth H, et al. Neurosci Lett. 2009 Oct 2;462(1):1-5. doi: 10.1016/j.neulet.2009.06.065. Epub 2009 Jun 25. Neurosci Lett. 2009. PMID: 19559752 Free PMC article.
References
- Agid Y, Ruberg M, Raisman-Vozari R, Hirsch EC, Javoy-Agid F (1990). The biochemistry of Parkinson’s disease. In: Parkinson’s disease (Stern G, ed.) pp. 99–125. London: Chapman and Hall.
- Ahmadi FA, Linseman DA, Grammatopoulos TN, Jones SM, Bouchard RJ, Freed CR, Heidenreich KA, Zawada WM (2003). The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J Neurochem 87:914–921. - PubMed
- Anglade P, Tsuji S, Javoy-Agid F, Agid Y, Hirsch EC (1995). Plasticity of nerve afferents to nigrostriatal neurons in Parkinson’s disease. Ann Neurol 37:265–272. - PubMed
- Ballard PA, Tetrud JW, Langston JW (1985). Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology 35:949–956. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous