The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis - PubMed (original) (raw)
The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis
Michael J Wolfgang et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Fatty acid synthesis in the central nervous system is implicated in the control of food intake and energy expenditure. An intermediate in this pathway, malonyl-CoA, mediates these effects. Malonyl-CoA is an established inhibitor of carnitine palmitoyltransferase-1 (CPT1), an outer mitochondrial membrane enzyme that controls entry of fatty acids into mitochondria and, thereby, fatty acid oxidation. CPT1c, a brain-specific enzyme with high sequence similarity to CPT1a (liver) and CPT1b (muscle) was recently discovered. All three CPTs bind malonyl-CoA, and CPT1a and CPT1b catalyze acyl transfer from various fatty acyl-CoAs to carnitine, whereas CPT1c does not. These findings suggest that CPT1c has a unique function or activation mechanism. We produced a targeted mouse knockout (KO) of CPT1c to investigate its role in energy homeostasis. CPT1c KO mice have lower body weight and food intake, which is consistent with a role as an energy-sensing malonyl-CoA target. Paradoxically, CPT1c KO mice fed a high-fat diet are more susceptible to obesity, suggesting that CPT1c is protective against the effects of fat feeding. CPT1c KO mice also exhibit decreased rates of fatty acid oxidation, which may contribute to their increased susceptibility to diet-induced obesity. These findings indicate that CPT1c is necessary for the regulation of energy homeostasis.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
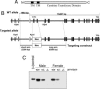
Comparison of amino acid sequence and functional properties of CPT1a and CPT1c. (A) Amino acid sequence identity and similarity comparison between CPT1a, CPT1b, and CPT1c. (B) Mitochondria from 293 T cells transfected with expression vectors for CPT1a, CPT1a (H473A), CPT1c, or CPT1c (H470A) were used in carnitine acyltransferase assays with palmitoyl-CoA (75 μM) as a substrate. “mg” refers to mitochondrial protein. (C) Concentration dependence of malonyl-CoA binding to CPT1c. (D) Comparison of malonyl-CoA binding to CPT1a and CPT1c.
Fig. 2.
Generation of CPT1c KO mice. (A) Predicted domain structure of CPT1c. TM, transmembrane domain. (B) Exons 1 and 2 containing the initiation codon and first transmembrane domain were targeted by homologous recombination in ES cells. Neo, neomycin-resistance cassette. (C) Western blots of whole-brain lysates of CPT1c WT and KO mice. KO mice expressed no detectable CPT1c, and heterozygous mice exhibited approximately half the expression of WT mice. “(+)control” refers to a FLAG-tagged CPT1c.
Fig. 3.
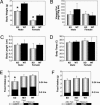
CPT1c KO mice are hypophagic and have a lower body weight than WT mice. (A) Body weight was significantly lower in CPT1c KO males (∗, P < 0.0001; n = 10 per group) and females (∗, P < 0.005; n = 10 per group). (B) CPT1c KO male mice have a trend toward increased visceral adipose tissue (n = 6 per group). The CPT1c KO does not affect body length (C) or temperature (D) (n = 6 per group). (E) CPT1c KO mice exhibit lower food intake than WT or heterozygous littermates (∗, P < 0.05; n = 6 per group) after a short (4-h) fast just before the dark cycle. (F) Effect of CPT1c KO on food intake after a 24-h fast. Error bars indicate SEM.
Fig. 4.
CPT1c KO mice are more susceptible to the effect of a high-fat diet. Male and female WT and CPT1c KO mice (n = 5 per group) were fed a control diet (10% of total kcal from fat; 1 kcal = 4.18 kJ) (A) or a high-fat diet (45% of total kcal from fat) (B) for 14 weeks. Animals were weighed weekly at 1400 hours. Initial body weights (average grams) were as follows: male WT, 25.9 g; male KO, 22.9 g; female WT, 20.9 g; and female KO, 19.0 g (∗, P < 0.05).
Fig. 5.
CPT1c KO mice exhibit mild insulin resistance when fed a high-fat diet. WT and CPT1c KO mice fed a high-fat diet for 17 weeks were subjected to an i.p. glucose tolerance test. CPT1c KO mice exhibited an elevated and prolonged plasma glucose level (A) and concomitant elevated plasma insulin level (B) indicative of insulin resistance (n = 5 per group).
Fig. 6.
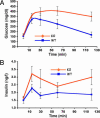
CPT1c KO mice exhibit lower fasting whole-body fatty acid oxidation. WT and CPT1c KO weight- and sex-matched mice were tested for their ability to β-oxidize [1-14C]oleic acid to 14CO2 at 1, 2, and 3 h after i.p. injection of labeled fatty acid. (A and C) Rate of 14CO2 production. (B and D) Total 14CO2 production. (A and B) In the ad libitum fed state, WT and CPT1c KO mice exhibited equivalent rates of fatty acid oxidation. (C and D) In the fasted state (24 h), CPT1c KO mice had significantly decreased fatty acid oxidation rates at 1, 2, and 3 h (C) and decreased total fasting fatty acid oxidation (D) (∗, P < 0.007).
Similar articles
- Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA.
Lane MD, Wolfgang M, Cha SH, Dai Y. Lane MD, et al. Int J Obes (Lond). 2008 Sep;32 Suppl 4:S49-54. doi: 10.1038/ijo.2008.123. Int J Obes (Lond). 2008. PMID: 18719599 Review. - Brain-specific carnitine palmitoyl-transferase-1c: role in CNS fatty acid metabolism, food intake, and body weight.
Wolfgang MJ, Cha SH, Millington DS, Cline G, Shulman GI, Suwa A, Asaumi M, Kurama T, Shimokawa T, Lane MD. Wolfgang MJ, et al. J Neurochem. 2008 May;105(4):1550-9. doi: 10.1111/j.1471-4159.2008.05255.x. Epub 2008 Jan 28. J Neurochem. 2008. PMID: 18248603 Free PMC article. - Metabolomic profiling reveals a role for CPT1c in neuronal oxidative metabolism.
Lee J, Wolfgang MJ. Lee J, et al. BMC Biochem. 2012 Oct 25;13:23. doi: 10.1186/1471-2091-13-23. BMC Biochem. 2012. PMID: 23098614 Free PMC article. - Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity.
Wolfgang MJ, Lane MD. Wolfgang MJ, et al. FEBS J. 2011 Feb;278(4):552-8. doi: 10.1111/j.1742-4658.2010.07978.x. Epub 2010 Dec 30. FEBS J. 2011. PMID: 21199367 Review. - Enhanced susceptibility of Cpt1c knockout mice to glucose intolerance induced by a high-fat diet involves elevated hepatic gluconeogenesis and decreased skeletal muscle glucose uptake.
Gao XF, Chen W, Kong XP, Xu AM, Wang ZG, Sweeney G, Wu D. Gao XF, et al. Diabetologia. 2009 May;52(5):912-20. doi: 10.1007/s00125-009-1284-0. Epub 2009 Feb 18. Diabetologia. 2009. PMID: 19224198
Cited by
- The role of hypothalamic H1 receptor antagonism in antipsychotic-induced weight gain.
He M, Deng C, Huang XF. He M, et al. CNS Drugs. 2013 Jun;27(6):423-34. doi: 10.1007/s40263-013-0062-1. CNS Drugs. 2013. PMID: 23640535 Review. - Molecular system bioenergetics: regulation of substrate supply in response to heart energy demands.
Saks V, Favier R, Guzun R, Schlattner U, Wallimann T. Saks V, et al. J Physiol. 2006 Dec 15;577(Pt 3):769-77. doi: 10.1113/jphysiol.2006.120584. Epub 2006 Sep 28. J Physiol. 2006. PMID: 17008367 Free PMC article. Review. - AMPK: a therapeutic target of heart failure-not only metabolism regulation.
Li X, Liu J, Lu Q, Ren D, Sun X, Rousselle T, Tan Y, Li J. Li X, et al. Biosci Rep. 2019 Jan 3;39(1):BSR20181767. doi: 10.1042/BSR20181767. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30514824 Free PMC article. Review. - MicroRNA-377-3p inhibits hepatocellular carcinoma growth and metastasis through negative regulation of CPT1C-mediated fatty acid oxidation.
Zhang T, Zhang Y, Liu J, Ma Y, Ye Q, Yan X, Ding L. Zhang T, et al. Cancer Metab. 2022 Jan 20;10(1):2. doi: 10.1186/s40170-021-00276-3. Cancer Metab. 2022. PMID: 35057851 Free PMC article. - Oleic acid directly regulates POMC neuron excitability in the hypothalamus.
Jo YH, Su Y, Gutierrez-Juarez R, Chua S Jr. Jo YH, et al. J Neurophysiol. 2009 May;101(5):2305-16. doi: 10.1152/jn.91294.2008. Epub 2009 Mar 4. J Neurophysiol. 2009. PMID: 19261705 Free PMC article.
References
- Dowell P., Hu Z., Lane M. D. Annu. Rev. Biochem. 2005;74:515–534. - PubMed
- Obici S., Feng Z., Morgan K., Stein D., Karkanias G., Rossetti L. Diabetes. 2002;51:271–275. - PubMed
- Loftus T. M., Jaworsky D. E., Frehywot G. L., Townsend C. A., Ronnett G. V., Lane M. D., Kuhajda F. P. Science. 2000;288:2379–2381. - PubMed
- Hu Z., Dai Y., Prentki M., Chohnan S., Lane M. D. J. Biol. Chem. 2005;280:39681–39683. - PubMed
- He W., Lam T. K., Obici S., Rossetti L. Nat. Neurosci. 2006;9:227–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials