Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells - PubMed (original) (raw)
Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells
Zhi Chen et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Suppressor of cytokine signaling (Socs) 3 is a cytokine-inducible inhibitor with critical but selective cell-specific effects. We show that deficiency of Socs3 in T cells had minimal effects on differentiation of T cells to the T helper (Th) 1 or Th2 subsets; accordingly, Socs3 had no effect on IL-12-dependent signal transducer and activator of transcription (Stat) 4 phosphorylation or IL-4-dependent Stat6 phosphorylation. By contrast, Socs3 was found to be a major regulator of IL-23-mediated Stat3 phosphorylation and Th17 generation, and Stat3 directly binds to the IL-17A and IL-17F promoters. We conclude that Socs3 is an essential negative regulator of IL-23 signaling, inhibition of which constrains the generation of Th17 differentiation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
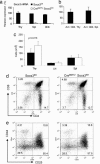
Fig. 1.
Socs3 is not essential for T cell development. Loss of Socs3 mRNA expression in thymocytes, splenocytes, isolated splenic CD4+ T cells (a), and activated CD4+ T cells (b) is shown. In these latter experiments, CD4+ T cells were isolated from thymi and spleen of Socs3fl/fl or CreMMTV Socs3fl/fl mice and activated by plate-bound anti-CD3 and anti-CD28 for 2 days. Total RNA extraction, cDNA synthesis, and real-time q-PCR were performed as described in Materials and Methods. Relative gene expression was normalized against the gene expression level of thymocytes from Socs3fl/fl mice. (c) Thymocytes, lymph node cells, and splenocytes were harvested and counted from 8- to 10-week-old CreMMTV Socs3fl/fl mice and their littermate controls. Data shown are the average from four mice. Student's t test was used to compare the data from CreMMTV Socs3fl/fl mice and their littermate controls. (d and e) Thymocytes from CreMMTV Socs3fl/fl mice and their littermate controls were stained with CD4-allophycocyanin and CD8-FITC. Thy1.2-positive, lineage-negative, CD4/8-double-negative thymocytes were stained for expression for CD25-FITC and CD44-allophycocyanin.
Fig. 2.
Socs3 deficiency has no significant effect on Th1 and Th2 differentiation. CD4+ T cells isolated from splenocytes of CreMMTV Socs3fl/fl and littermate control mice were cultured under Th1 or Th2 polarizing conditions for 7 days. (a and b) IFNγ- and IL-4-producing cells were detected by intracellular cytokine staining. Histograms are representative of four separate experiments. (c) IFNγ or IL-4 secreted by Th1 and Th2 cells from CreMMTV Socs3fl/fl and littermate control mice were measured from the cell culture supernatants by ELISA.
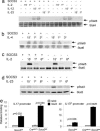
Fig. 3.
Socs3 selectively regulates Stat3 phosphorylation and binding to IL-17 and IL-17F promoters. Splenic CD4+ T cells isolated from CreMMTV Socs3fl/fl and littermate mice were activated with plate-bound anti-CD3 and anti-CD28 for 2 days, expanded in IL-2-containing media, rested, and restimulated with IL-2, IL-4, IL-12, or IL-23 for 15 min to 6 h. Cell lysates were separated by SDS/PAGE, transferred to nitrocellulose membranes, and immunoblotted for phospho-Stat6, phospho-Stat3, and phospho-Stat4. Protein loading was assessed by blotting with anti-β-actin and reprobing the membranes with pan-Stat antibodies. Data in a_–_d are representative of two to three independent experiments. Lysates were from wild-type and CreMMTV Socs3fl/fl CD4+ lymphoblasts stimulated with IL-23 for 1 h. Stat3 binding to the IL-17A and IL-17F promoter regions was assessed by chromatin immunoprecipitation. The DNA eluted from Stat3 precipitated samples was quantified by q-PCR. (e) Values were normalized to input value and are expressed as fold enrichment relative to normal rabbit serum (NRS) for each experiment.
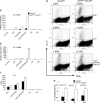
Fig. 4.
Socs3 negatively regulates IL-17 and IL-17F expression in T cells. Naïve splenic CD4+ T cells were enriched from CreMMTV Socs3fl/fl and littermate control mice and activated with plate-bound anti-CD3 and anti-CD28. (a and b) Cells were cultured in media containing Il-2 (40 international units/ml) under the following polarizing conditions: Th0 (no additional cytokines), Th1 [IL-12 (10 ng/ml) and anti-IL-4 (10 μg/ml)]; Th2 [IL-4 (10 ng;ml) and anti-IFNγ (10 μg/ml)]; and Il-23 (10 ng/ml) in the absence or presence of anti-IFNγ (10 μg/ml) or Th17 [IL-23 (10 ng/ml), anti-IFNγ (10 μg/ml), and anti-IL-4 (10 μg/ml)]. (c) IL-17A or IL-17F mRNA expression was detected by real-time q-PCR. (d and e) IL-17 protein production was measured in cell culture supernatants by ELISA and by intracellular cytokine staining using flow cytometry. Data shown are representative of two to four independent experiments.
References
- Brender C., Nielsen M., Kaltoft K., Mikkelsen G., Zhang Q., Wasik M., Billestrup N., Odum N. Blood. 2001;97:1056–1062. - PubMed
- Davey H. W., McLachlan M. J., Wilkins R. J., Hilton D. J., Adams T. E. Mol. Cell. Endocrinol. 1999;158:111–116. - PubMed
- Alexander W. S., Hilton D. J. Annu. Rev. Immunol. 2004;22:503–529. - PubMed
- O'Shea J. J., Gadina M., Schreiber R. D. Cell. 2002;109(Suppl):S121–S131. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous