NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis - PubMed (original) (raw)
Comparative Study
. 2006 May-Jun;13(3):307-15.
doi: 10.1101/lm.76006. Epub 2006 May 16.
Affiliations
- PMID: 16705139
- PMCID: PMC1475811
- DOI: 10.1101/lm.76006
Comparative Study
NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis
Kazuhiro Shimazu et al. Learn Mem. 2006 May-Jun.
Abstract
In the adult brain, the expression of NT-3 is largely confined to the hippocampal dentate gyrus (DG), an area exhibiting significant neurogenesis. Using a conditional mutant line in which the NT-3 gene is deleted in the brain, we investigated the role of NT-3 in adult neurogenesis, hippocampal plasticity, and memory. Bromodeoxyuridine (BrdU)-labeling experiments demonstrated that differentiation, rather than proliferation, of the neuronal precursor cells (NPCs) was significantly impaired in DG lacking NT-3. Triple labeling for BrdU, the neuronal marker NeuN, and the glial marker GFAP indicated that NT-3 affects the number of newly differentiated neurons, but not glia, in DG. Field recordings revealed a selective impairment in long-term potentiation (LTP) in the lateral, but not medial perforant path-granule neuron synapses. In parallel, the NT-3 mutant mice exhibited deficits in spatial memory tasks. In addition to identifying a novel role for NT-3 in adult NPC differentiation in vivo, our study provides a potential link between neurogenesis, dentate LTP, and spatial memory.
Figures
Figure 1.
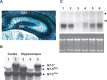
Cre-mediated recombination and ablation of the NT-3 gene in adult hippocampus. (A) Nestin-Cre mediated recombination in adult hippocampus (2-mo old). Coronal section from P2.5 animal [Nes-Cre/+(male) X floxedROSA-26ref/+(female)] stained by X-gal (dark blue) to detect cre-mediated recombination at the ROSA-26 locus and counterstained with Neutral red. Note the widespread and complete or near-complete recombination in hippocampus and cerebral cortex. (B) Southern blot showing Cre-mediated recombination in the hippocampus and cortex of adult mutant mice. DNA samples were prepared from brain tissues of mice of different genotypes. (Lane 1) NT-31lox/2lox, Nes-cre+/0; (lane 2) NT-32lox/+, Nes-cre+/0; (lane 3) NT-31lox/2lox, Nes-cre0/0; (lane 4) NT-31lox/2lox, Nes-cre+/0; (lane 5) NT-32lox/+, Nes-cre+/0. Arrows: +: Wild-type allele, or NT-3+; f: Floxed allele, or NT-32lox; R: knockout allele, or NT-31lox. (C) Northern blot analysis of mRNAs isolated from adult hippocampi of different genotypes. (Lane 1) NT-3+/+, Nes-cre0/0; (lane 2) NT-3+/+, Nescre+/0; (lane 3) NT-31lox/2lox, Nes-cre+/0; (lane 4) NT-31lox/2lox, Nes-cre0/0 ; (lane 5) NT-3+/−, Nes-cre0/0; (lane 6) NT-32lox/+, Nes-cre+/0. (Bottom) rRNA loading control is shown below. Arrows point to the positions of 18S and 28 S rRNAs.
Figure 2.
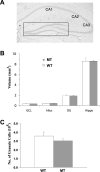
Normal morphology of adult hippocampus in NT-3 mutant mice. (A) Low power (4×) images of hippocampus from an NT-3 mutant mouse. H&E staining reveals no abnormality of morphology of the hippocampus. (B) Quantitative measurement of the volumes of the subregions of hippocampus. The volumes of granule cell layer (GCL), the hilar region, the whole dentate gyrus (DG), and the whole hippocampus were measured. n = 6 pairs of male mice; 2-mo old. (C) Summary of cell counts. Total number of granule cells in DG is presented. n = 5 pairs of 2-mo-old male mice.
Figure 3.
Effects of NT-3 deletion on proliferation and survival of NPC. (A) Medium-power (20×) images of BrdU-labeled cells in the wild-type (top) and mutant (bottom) dentate gyrus. The sections were processed for BrdU immunohistochemistry followed by Nissl counter stain. (B) Effect on proliferation of NPCs. Three hours after BrdU was injected intraperitoneally, the brains of the injected animals were fixed and processed for BrdU histochemistry. The number of BrdU (+) cells in the hilus and granule cell layer (GCL) were counted using sterological techniques. n = 3 pairs of male mice; 4-mo old. (C) Effect on survival of NPCs. BrdU was injected twice daily for 10 d, and BrdU immunohistochemistry was performed 2 wk after the last BrdU injection. Cells were counted the same way as B. n = 6 pairs of male mice; 2-mo old.
Figure 4.
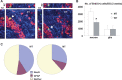
Effect of NT-3 deletion on NPC-derived neurons and glia. Wild-type and mutant mice were injected with BrdU twice/day for 10 d. Two weeks later, the animals were processed for triple labeling for BrdU (green), NeuN (red), and GFAP (blue), using confocal microscopy. (A) Confocal images of BrdU-positive neurons (left) and astroglia (right). (B) Quantitative analysis of the number of NPC-derived neurons (left) and that of glia (right) in the dentate of control and mutant mice. n = 6 pairs of male mice; 2-mo old. (*) Significantly different between control and mutant mice, P < 0.01, Student’s _t_-test, two-tailed. (C) Relative distribution of cells derived from NPCs. n = 6 pairs of male mice; 2-mo old.
Figure 5.
NT-3 regulates synaptic plasticity at a subset of hippocampal synapses. (A) Schematic showing the arrangement of stimulating and recording electrodes in CA1 (top) and DG (bottom). (B) LTP recordings in the CA1 region (top), the lateral perforant path (LPP) (middle), and the medial perforant path (MPP) (bottom). Tetanic stimulation was applied at time “0”. Data from multiple slices (n) were pooled and expressed as mean ± SEM. (C) LTP deficit in LPP synapses in the presence of GABAa antagonist bicuculline (10 μM). Mice with the NT-3 gene floxed (NT-32lox/2lox, Nes-cre0/0, indicated as flox/flox) were studied as an additional control. Number of mice (N) and slices (n) are indicated in the figure. All mice used in electrophysiology and behavior experiments were between 1.5 and 2.5-mo old.
Figure 6.
Impairments in presynaptic function of LPP-dentate synapses in NT-3 mutant mice. Numbers of slices recorded are indicated in the respective figures. (A) Input–output curves. No difference was observed between control and mutant mice. (B) Paired pulse facilitation (PPF). NT-3 mutant mice exhibit a substantial reduction in PPF at shorter interstimulus intervals. (C) Post-tetanic potentiation (PTP). PTP was induced by a tetanus (100 Hz, 1 sec) in the presence of NMDA receptor blocker (Apv, 100 μM). There was a small, but significant decrease in PTP in the NT-3 mutant mice. (D) Synaptic fatigue. Synaptic responses to a brief, high-frequency stimulation (HFS, 100 Hz, 20 pulses) were recorded. The slopes of all EPSPs were normalized to that of the 2nd EPSP. (E) Synaptic response to a prolonged, repetitive stimulation (LFS, 300 stimuli at 14 Hz, 280 pulses).
Figure 7.
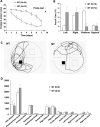
Deficits in spatial learning and memory in NT-3 mutant mice. (A) Deficits in acquisition. The animals were trained twice a day for 7 d to find the hidden platform. The latency to find the platform is plotted against the training days. The wild-type mice rapidly learned the location of the submerged platform, whereas the mutant mice showed a much slower learning curve (ANOVA, P < 0.05). (B) Deficits in spatial memory. The probe test conducted at the end of training period. The platform was removed and the animals were allowed to swim freely in the pool for 60 sec. The percentage of time the animals spent in each of the four quadrants was measured. The mutant mice spent less time in the platform quadrant than wild-type mice (ANOVA followed by post hoc test, P < 0.002). (C) Representative swim paths illustrate the impairment of spatial cognition during the probe trial in the mutant mice. (D) Open field activity. Locomotor activities were measured every 10 msec for 10 min. Unit of measurements: time, sec; distance, cm; beam break, number of times.
Similar articles
- Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis.
Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Zhao M, et al. Biol Psychiatry. 2007 Sep 1;62(5):381-90. doi: 10.1016/j.biopsych.2006.10.019. Epub 2007 Jan 18. Biol Psychiatry. 2007. PMID: 17239352 - Conditional reduction of adult neurogenesis impairs bidirectional hippocampal synaptic plasticity.
Massa F, Koehl M, Wiesner T, Grosjean N, Revest JM, Piazza PV, Abrous DN, Oliet SH. Massa F, et al. Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6644-9. doi: 10.1073/pnas.1016928108. Epub 2011 Apr 4. Proc Natl Acad Sci U S A. 2011. PMID: 21464314 Free PMC article. - Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus.
Snyder JS, Kee N, Wojtowicz JM. Snyder JS, et al. J Neurophysiol. 2001 Jun;85(6):2423-31. doi: 10.1152/jn.2001.85.6.2423. J Neurophysiol. 2001. PMID: 11387388 - Stem cell review series: role of neurogenesis in age-related memory disorders.
Drapeau E, Nora Abrous D. Drapeau E, et al. Aging Cell. 2008 Aug;7(4):569-89. doi: 10.1111/j.1474-9726.2008.00369.x. Epub 2008 Jan 23. Aging Cell. 2008. PMID: 18221417 Free PMC article. Review.
Cited by
- Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice.
Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Marlatt MW, et al. Dev Neurobiol. 2012 Jun;72(6):943-52. doi: 10.1002/dneu.22009. Dev Neurobiol. 2012. PMID: 22252978 Free PMC article. - The adaptive significance of adult neurogenesis: an integrative approach.
Konefal S, Elliot M, Crespi B. Konefal S, et al. Front Neuroanat. 2013 Jul 16;7:21. doi: 10.3389/fnana.2013.00021. eCollection 2013. Front Neuroanat. 2013. PMID: 23882188 Free PMC article. - Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice.
Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Fuss J, et al. PLoS One. 2010 Sep 16;5(9):e12769. doi: 10.1371/journal.pone.0012769. PLoS One. 2010. PMID: 20862278 Free PMC article. - From neurotransmitters to neurotrophic factors to neurogenesis.
Hagg T. Hagg T. Neuroscientist. 2009 Feb;15(1):20-7. doi: 10.1177/1073858408324789. Neuroscientist. 2009. PMID: 19218228 Free PMC article. Review. - Whole-genome sequencing reveals KRTAP1-1 as a novel genetic variant associated with antidepressant treatment outcomes.
Park JH, Lim SW, Myung W, Park I, Jang HJ, Kim S, Lee MS, Chang HS, Yum D, Suh YL, Kim JW, Kim DK. Park JH, et al. Sci Rep. 2021 Feb 25;11(1):4552. doi: 10.1038/s41598-021-83887-6. Sci Rep. 2021. PMID: 33633223 Free PMC article.
References
- Abraham W.C., Williams J.M. Properties and mechanisms of LTP maintenance. Neuroscientist. 2003;9:463–474. - PubMed
- Akbarian S., Bates B., Liu R.J., Skirboll S.L., Pejchal T., Coppola V., Sun L.D., Fan G., Kucera J., Wilson M.A., et al. Neurotrophin-3 modulates noradrenergic neuron function and opiate withdrawal. Mol. Psychiatry. 2001;6:593–604. - PubMed
- Asztely F., Kokaia M., Olofsdotter K., Ortegren U., Lindvall O. Afferent-specific modulation of short-term synaptic plasticity by neurotrophins in dentate gyrus. Eur. J. Neurosci. 2000;12:662–669. - PubMed
- Bates B., Rios M., Trumpp A., Chen C., Fan G., Bishop J.M., Jaenisch R. Neurotrophin-3 is required for proper cerebellar development. Nat. Neurosci. 1999;2:115–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous