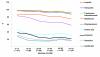Multidrug-resistant commensal Escherichia coli in children, Peru and Bolivia - PubMed (original) (raw)
doi: 10.3201/eid1206.051258.
Lucia Pallecchi, Marta Benedetti, Connie Fernandez, Yolanda Vallejos, Elisa Guzman, Ana Liz Villagran, Antonia Mantella, Chiara Lucchetti, Filippo Bartalesi, Marianne Strohmeyer, Angela Bechini, Herlan Gamboa, Hugo Rodríguez, Torkel Falkenberg, Göran Kronvall, Eduardo Gotuzzo, Franco Paradisi, Gian Maria Rossolini
Affiliations
- PMID: 16707045
- PMCID: PMC3373029
- DOI: 10.3201/eid1206.051258
Multidrug-resistant commensal Escherichia coli in children, Peru and Bolivia
Alessandro Bartoloni et al. Emerg Infect Dis. 2006 Jun.
Abstract
Using a rapid screening method, we investigated the prevalence of fecal carriage of antimicrobial drug-resistant Escherichia coli in 3,174 healthy children from 4 urban settings in Peru and Bolivia. High resistance rates were observed for ampicillin (95%), trimethoprim-sulfamethoxazole (94%), tetracycline (93%), streptomycin (82%), and chloramphenicol (70%). Lower resistance rates were observed for nalidixic acid (35%), kanamycin (28%), gentamicin (21%), and ciprofloxacin (18%); resistance to ceftriaxone and amikacin was uncommon (<0.5%). In a random sample of 1,080 resistant E. coli isolates, 90% exhibited a multidrug-resistance (MDR) phenotype. The 2 most common MDR phenotypes (ampicillin/tetracycline/trimethoprim-sulfamethoxazole and ampicillin/tetracycline/trimethoprim-sulfamethoxazole/chloramphenicol) could be transferred en bloc in conjugation experiments. The most common acquired resistance genes were blaTEM, tet(A), tet(B), drfA8, sul1, sul2, and catI. These findings underscore the magnitude of the problem of antimicrobial drug resistance in low-resource settings and the urgent need for surveillance and control of this phenomenon.
Figures
Figure 1
Flow chart of microbiologic analysis of fecal samples. DPM, direct plating method.
Figure 2
Total prevalence, by age group, of fecal carriage of antimicrobial drug–resistant Escherichia coli among 3,174 children in 4 urban areas of Bolivia and Peru. Ceftriaxone and amikacin were not considered in these analyses because their resistance rates were too low.
Figure 3
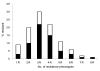
Frequency of resistance phenotypes in 1,080 randomly selected antimicrobial drug–resistant Escherichia coli isolates from 4 urban areas of Bolivia and Peru. Black bars indicate the most frequent resistance and multidrug-resistance phenotype within each category: 1R, TET; 2R, AMP-SXT; 3R, AMP-TET-SXT; 4R, AMP-TET-SXT-CHL; 5R, AMP-TET-SXT-CHL-KAN; 6R, AMP-TET-SXT-CHL-NAL-CIP; 7R, AMP-TET-SXT-CHL-GEN-NAL-CIP; 8R, AMP-TET-SXT-CHL-KAN-GEN-NAL-CIP. AMP, ampicillin; TET, tetracycline; SXT, trimethoprim-sulfamethoxazole; CHL, chloramphenicol; KAN, kanamycin; GEN, gentamicin; NAL, nalidixic acid; CIP, ciprofloxacin.
Similar articles
- Phenotypic and genotypic characterization of antimicrobial resistance in Escherichia coli O111 isolates.
Guerra B, Junker E, Schroeter A, Helmuth R, Guth BE, Beutin L. Guerra B, et al. J Antimicrob Chemother. 2006 Jun;57(6):1210-4. doi: 10.1093/jac/dkl127. Epub 2006 Apr 7. J Antimicrob Chemother. 2006. PMID: 16603644 - Carriage of antibiotic-resistant Escherichia coli among healthy children and home-raised chickens: a household study in a resource-limited setting.
Riccobono E, Pallecchi L, Mantella A, Bartalesi F, Zeballos IC, Trigoso C, Villagran AL, Bartoloni A, Rossolini GM. Riccobono E, et al. Microb Drug Resist. 2012 Feb;18(1):83-7. doi: 10.1089/mdr.2011.0003. Epub 2011 Jun 28. Microb Drug Resist. 2012. PMID: 21711148 - Acquired sulphonamide resistance genes in faecal Escherichia coli from healthy children in Bolivia and Peru.
Infante B, Grape M, Larsson M, Kristiansson C, Pallecchi L, Rossolini GM, Kronvall G. Infante B, et al. Int J Antimicrob Agents. 2005 Apr;25(4):308-12. doi: 10.1016/j.ijantimicag.2004.12.004. Int J Antimicrob Agents. 2005. PMID: 15784310 - Molecular characterization of antimicrobial resistance in enterococci and Escherichia coli isolates from European wild rabbit (Oryctolagus cuniculus).
Silva N, Igrejas G, Figueiredo N, Gonçalves A, Radhouani H, Rodrigues J, Poeta P. Silva N, et al. Sci Total Environ. 2010 Sep 15;408(20):4871-6. doi: 10.1016/j.scitotenv.2010.06.046. Epub 2010 Jul 10. Sci Total Environ. 2010. PMID: 20624632 - Prevalence of antimicrobial resistance and resistance genes in faecal Escherichia coli isolates recovered from healthy pets.
Costa D, Poeta P, Sáenz Y, Coelho AC, Matos M, Vinué L, Rodrigues J, Torres C. Costa D, et al. Vet Microbiol. 2008 Feb 5;127(1-2):97-105. doi: 10.1016/j.vetmic.2007.08.004. Epub 2007 Aug 15. Vet Microbiol. 2008. PMID: 17870255
Cited by
- Antibiotic-Resistant Escherichia coli in Drinking Water Samples from Rural Andean Households in Cajamarca, Peru.
Larson A, Hartinger SM, Riveros M, Salmon-Mulanovich G, Hattendorf J, Verastegui H, Huaylinos ML, Mäusezahl D. Larson A, et al. Am J Trop Med Hyg. 2019 Jun;100(6):1363-1368. doi: 10.4269/ajtmh.18-0776. Am J Trop Med Hyg. 2019. PMID: 31017079 Free PMC article. - Evidence of Community-Wide Spread of Multi-Drug Resistant Escherichia coli in Young Children in Lusaka and Ndola Districts, Zambia.
Bumbangi FN, Llarena AK, Skjerve E, Hang'ombe BM, Mpundu P, Mudenda S, Mutombo PB, Muma JB. Bumbangi FN, et al. Microorganisms. 2022 Aug 21;10(8):1684. doi: 10.3390/microorganisms10081684. Microorganisms. 2022. PMID: 36014101 Free PMC article. - Risk factors for antibiotic-resistant Escherichia coli carriage in young children in Peru: community-based cross-sectional prevalence study.
Kalter HD, Gilman RH, Moulton LH, Cullotta AR, Cabrera L, Velapatiño B. Kalter HD, et al. Am J Trop Med Hyg. 2010 May;82(5):879-88. doi: 10.4269/ajtmh.2010.09-0143. Am J Trop Med Hyg. 2010. PMID: 20439971 Free PMC article. - Access to health care in relation to socioeconomic status in the Amazonian area of Peru.
Kristiansson C, Gotuzzo E, Rodriguez H, Bartoloni A, Strohmeyer M, Tomson G, Hartvig P. Kristiansson C, et al. Int J Equity Health. 2009 Apr 15;8:11. doi: 10.1186/1475-9276-8-11. Int J Equity Health. 2009. PMID: 19368731 Free PMC article. - Antimicrobial resistance genes are enriched in aerosols near impacted urban surface waters in La Paz, Bolivia.
Ginn O, Nichols D, Rocha-Melogno L, Bivins A, Berendes D, Soria F, Andrade M, Deshusses MA, Bergin M, Brown J. Ginn O, et al. Environ Res. 2021 Mar;194:110730. doi: 10.1016/j.envres.2021.110730. Epub 2021 Jan 11. Environ Res. 2021. PMID: 33444611 Free PMC article.
References
- World Health Organization. WHO report on infectious diseases 2000. Overcoming antimicrobial resistance. Geneva: The Organization; 2000.
- World Health Organization. Global strategy for containment of antimicrobial resistance. Geneva: The Organization; 2001. [cited 2006 Jan 27]. Available from http://www.who.int/drugresistance/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials