Presenilin-dependent gamma-secretase-mediated control of p53-associated cell death in Alzheimer's disease - PubMed (original) (raw)
Presenilin-dependent gamma-secretase-mediated control of p53-associated cell death in Alzheimer's disease
Cristine Alves da Costa et al. J Neurosci. 2006.
Abstract
Presenilins (PSs) are part of the gamma-secretase complex that produces the amyloid beta-peptide (Abeta) from its precursor [beta-amyloid precursor protein (betaAPP)]. Mutations in PS that cause familial Alzheimer's disease (FAD) increase Abeta production and trigger p53-dependent cell death. We demonstrate that PS deficiency, catalytically inactive PS mutants, gamma-secretase inhibitors, and betaAPP or amyloid precursor protein-like protein 2 (APLP2) depletion all reduce the expression and activity of p53 and lower the transactivation of its promoter and mRNA expression. p53 expression also is diminished in the brains of PS- or betaAPP-deficient mice. The gamma- and epsilon-secretase-derived amyloid intracellular C-terminal domain (AICD) fragments (AICDC59 and AICDC50, respectively) of betaAPP trigger p53-dependent cell death and increase p53 activity and mRNA. Finally, PS1 mutations enhance p53 activity in human embryonic kidney 293 cells and p53 expression in FAD-affected brains. Thus our study shows that AICDs control p53 at a transcriptional level, in vitro and in vivo, and that FAD mutations increase p53 expression and activity in cells and human brains.
Figures
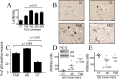
Figure 1.
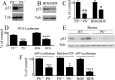
Influence of PS deficiency on p53 in vitro and in vivo. A, B, p53 immunoreactivity in nuclear extracts of wild-type (PS+/+, BD6) or PS-deficient (PS−/−, BD8) fibroblasts (A) or blastocysts (B). C, The bars correspond to densitometric analyses of p53 in the indicated cell line expressed as a percentage of p53 immunoreactivity in the corresponding wild-type cell line and are the means ± SEM of three independent determinations. D, p53 activity assessed by transient cotransfection of β-galactosidase and PG13-luciferase cDNA in the indicated fibroblast and blastocyst cell lines as described in Materials and Methods. Bars correspond to the ratios of luciferase/β-galactosidase activities (see Materials and Methods) expressed as a percentage of control activities monitored in PS+/+ or BD6 and are the means ± SEM of six independent determinations. E, p53 immunoreactivity measured in whole proteic extracts prepared from wild-type (WT) or double-conditional PS knock-out (PS−/−) mice brains. F, Transactivation of the p53 promoter and real-time PCR analysis of p53 mRNA. p53 promoter transactivation was measured after transfection of the mPP-luciferase construct in the indicated fibroblast and blastocyst cell lines. Bars correspond to the ratios of luciferase/β-galactosidase activities expressed as a percentage of control activities monitored in PS+/+ or BD6 and are the means ± SEM of six independent determinations. Real-time PCR analysis was performed in the indicated fibroblastic cell lines. Bars correspond to the means ± SEM of three independent determinations. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.0005; ∗∗∗∗p < 0.0001.
Figure 2.
Effect of γ-secretase inhibitors on p53. PS+/+ and PS−/− fibroblasts were transiently transfected with PG13-luciferase or mPP-luciferase vectors as described in Materials and Methods. A–C, At 24 h after transfection, the cells were treated for 24 h without (Ct) or with the indicated γ-secretase inhibitor (DFK167, 50 μ
m
; L685, 2 μ
m
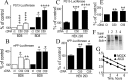
); then p53 expression in nuclear extracts (A), p53 transcriptional activity (B), and transactivation of mPP (C) were measured as described in Materials and Methods. Bars correspond to the ratios of luciferase/β-galactosidase activities expressed as a percentage of control activities monitored in PS+/+ and are the means ± SEM of three independent determinations. D–F, Stably transfected TSM1 neuronal cells overexpressing Asp257Ala-PS1 (PS1-257) or Asp385Ala-PS1 (PS1-385) were assayed for their susceptibility to staurosporine-induced caspase 3 activation (E) and were analyzed for their p53 mRNA levels by real-time PCR (F) as described in Materials and Methods. Bars in E and F are the means ± SEM of nine and three independent experiments, respectively, performed in duplicate (E) or triplicate (F). ∗p < 0.01; ∗∗p < 0.001.
Figure 3.
AICDC50 and AICDC59 modulate p53. A–D, BD6 and BD8 blastocysts (A, B) or HEK 293 cells (C, D) were transiently transfected with PG13-luciferase (A, C), mPP-luciferase (B), or hPP-luciferase (D) vectors together with an empty vector (Ct), AICDC50 (C50), or AICDC59 (C59) cDNAs as described in Materials and Methods. At 48 h after transfection, the p53 transcriptional activity (A, C) or transactivation of mPP (B) or hPP (D) was measured as detailed in Materials and Methods. Bars correspond to the ratios of luciferase/β-galactosidase activities expressed as a percentage of control activities (taken as 100) in corresponding cells transfected with empty vector and are the means ± SEM of 12–18 independent determinations. E, HEK 293 cells were transiently transfected with empty vector (Ct), AICDC50 (C50), or AICDC59 (C59) cDNA; then p53 mRNA levels were analyzed 48 h after transfection by real-time PCR as described in Materials and Methods. Bars correspond to p53 mRNA density expressed as a percentage of control mock-transfected cells (taken as 100) and are the means ± SEM of three independent experiments. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.005; ∗∗∗∗p < 0.001. F, Gel shift analysis obtained after transient cotransfection of HEK 293 cells with AICDC59, Tip60, and Fe65 cDNAs as described in Materials and Methods. Lane 1, 32P-labeled p53-2 oligo retarded by AICD59-enriched nuclear extracts; lane 2, 32P-labeled p53-2 probe in competition with cold p53-2 probe; lane 3, supershift of the 32P-labeled p53-2 probe by anti-myc antibodies; lane 4, supershift of the 32P-labeled p53-2 probe with control nonspecific anti-mouse antibody. G, The kinetics of p53 mRNA level decreases in actinomycin D-treated mock- and AICDC59-transfected HEK 293 cells. At 33 h after transfection the cells were treated for the indicated times with 10 μg/ml of actinomycin D; then p53 mRNA levels were estimated by real-time PCR as described in Materials and Methods.
Figure 4.
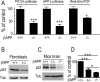
AICDC59 increases caspase 3 activity in a p53-dependent manner. Wild-type fibroblasts were transfected with pcDNA3 or AICDC59 coding vectors cotransfected or not (Ct) with Tip60 and Fe65 cDNA. A, AICD, Fe65, and Tip60 immunoreactivities were analyzed as described in Materials and Methods. B, Caspase 3 activity was measured after stimulation with staurosporine (see Materials and Methods). Cells were treated with pifithrin-α (black bars) or a pifithrin-α-inactive analog (gray bars) or were not treated (white bars). C, Pifithrin-α-sensitive p53-dependent caspase 3 activation was determined by the difference between the caspase 3 activities measured in the absence and in the presence of the inhibitor. Bars in B and C are the means ± SEM of three to six independent determinations. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, not significant.
Figure 5.
Influence of βAPP depletion on p53 in vitro and in vivo. A, p53 transcriptional activity, transactivation of murine p53 promoter, and p53 mRNA levels were measured as described in Materials and Methods in wild-type βAPP (+/+) or βAPP-deficient (−/−) fibroblasts. Bars for PG13- and mPP-luciferase activities correspond to the ratios of luciferase/β-galactosidase activities expressed as a percentage of control activities monitored in βAPP+/+ and are the means ± SEM of six independent determinations. Bars corresponding to real-time PCR analysis of p53 mRNA are the means ± SEM of four independent determinations. p53-like immunoreactivity in the indicated cell lines (B) or in extracts from the indicated mice brain (C) was measured as described in Materials and Methods. Bars in D correspond to densitometric analyses of p53 in the indicated mice brain and are the means ± SEM of three independent determinations. ∗p < 0.05; ∗∗∗p < 0.001.
Figure 6.
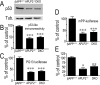
Influence of APLP2 depletion on p53. p53 immunoreactivity (A, B), p53 activity (C), transactivation of murine p53 promoter (D), and mRNA levels (E) were measured as described in Materials and Methods in wild-type (βAPP+/+), APLP2-deficient (APLP2−/−), or double βAPP/APLP2-deficient (DKO) fibroblasts. Note that fibroblasts do not express APLP1 (data not shown). Bars in B–D correspond to the densitometric analysis of p53 immunoreactivity (B) or to the ratios of luciferase/β-galactosidase activities (C, D) expressed as a percentage of control monitored in βAPP+/+ and are the means ± SEM of six independent determinations. Bars in E correspond to the mRNA levels measured by real-time PCR, are expressed as a percentage of mRNA levels in βAPP+/+ fibroblasts, and are the means ± SEM of four experiments. ∗p < 0.01; ∗∗p < 0.005, ∗∗∗p < 0.001.
Figure 7.
Influence of PS1 mutations on p53 expression in FAD-affected human brains. A, p53 transcriptional activity after transient transfection of HEK 293 cells with empty cDNA (Ct) or PS1 cDNA harboring the M146V, (146), H163R (163), G209V (209), or PS1ΔE9 (ΔE9) mutations and deletion. Bars correspond to the ratios of luciferase/β-galactosidase activities expressed as a percentage of control activities in cells transfected with empty vector and are the means ± SEM of three independent determinations. B, p53 immunohistochemistry in temporal cortex of control subjects (Ct, top panels) and FAD cases (bottom panels; see cases in Materials and Methods). Note very few neuronal labels in control case (top left panel), with a faint label in microglial cells (arrow in top right panel), p53 immunolabeling in the cytoplasm of neurons in FAD (arrowheads in bottom left panel), and p53-like immunoreactivity associated with cotton wool plaque in FAD (arrows in bottom right panel). Scale bars, 20 μm. C, The bars correspond to the number of p53-positive neurons in controls, sporadic AD, and FAD brains (see Materials and Methods) and are the means ± SEM. D, Values correspond to the densitometric analysis of p53-like immunoreactivity in controls, sporadic AD, and FAD brains (see Materials and Methods). Inset represents a typical Western blot analysis comparing p53-like immunoreactivity in control (Ct) and FAD brain. E, Values correspond to the densitometric analysis of IDE-like immunoreactivity measured as detailed in Materials and Methods in controls, sporadic AD, and FAD brains. ns, Not statistically significant when compared with controls.
Similar articles
- The gamma/epsilon-secretase-derived APP intracellular domain fragments regulate p53.
Checler F, Sunyach C, Pardossi-Piquard R, Sévalle J, Vincent B, Kawarai T, Girardot N, St George-Hyslop P, da Costa CA. Checler F, et al. Curr Alzheimer Res. 2007 Sep;4(4):423-6. doi: 10.2174/156720507781788945. Curr Alzheimer Res. 2007. PMID: 17908046 Review. - Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.
Steiner H, Capell A, Leimer U, Haass C. Steiner H, et al. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review. - NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42.
Buggia-Prevot V, Sevalle J, Rossner S, Checler F. Buggia-Prevot V, et al. J Biol Chem. 2008 Apr 11;283(15):10037-47. doi: 10.1074/jbc.M706579200. Epub 2008 Feb 8. J Biol Chem. 2008. PMID: 18263584 - p53-dependent control of transactivation of the Pen2 promoter by presenilins.
Dunys J, Sevalle J, Giaime E, Pardossi-Piquard R, Vitek MP, Renbaum P, Levy-Lahad E, Zhang YW, Xu H, Checler F, da Costa CA. Dunys J, et al. J Cell Sci. 2009 Nov 1;122(Pt 21):4003-8. doi: 10.1242/jcs.051169. J Cell Sci. 2009. PMID: 19889971 Free PMC article. - Familial Alzheimer's disease mutations inhibit gamma-secretase-mediated liberation of beta-amyloid precursor protein carboxy-terminal fragment.
Wiley JC, Hudson M, Kanning KC, Schecterson LC, Bothwell M. Wiley JC, et al. J Neurochem. 2005 Sep;94(5):1189-201. doi: 10.1111/j.1471-4159.2005.03266.x. Epub 2005 Jun 30. J Neurochem. 2005. PMID: 15992373
Cited by
- Nuclear factor-κB regulates βAPP and β- and γ-secretases differently at physiological and supraphysiological Aβ concentrations.
Chami L, Buggia-Prévot V, Duplan E, Del Prete D, Chami M, Peyron JF, Checler F. Chami L, et al. J Biol Chem. 2012 Jul 13;287(29):24573-84. doi: 10.1074/jbc.M111.333054. Epub 2012 May 31. J Biol Chem. 2012. PMID: 22654105 Free PMC article. - Model Hirano bodies protect against tau-independent and tau-dependent cell death initiated by the amyloid precursor protein intracellular domain.
Furgerson M, Fechheimer M, Furukawa R. Furgerson M, et al. PLoS One. 2012;7(9):e44996. doi: 10.1371/journal.pone.0044996. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028730 Free PMC article. - Comprehensive MicroRNAome Analysis of the Relationship Between Alzheimer Disease and Cancer in PSEN Double-Knockout Mice.
Ham S, Kim TK, Ryu J, Kim YS, Tang YP, Im HI. Ham S, et al. Int Neurourol J. 2018 Dec;22(4):237-245. doi: 10.5213/inj.1836274.137. Epub 2018 Dec 31. Int Neurourol J. 2018. PMID: 30599494 Free PMC article. - TMP21 transmembrane domain regulates gamma-secretase cleavage.
Pardossi-Piquard R, Böhm C, Chen F, Kanemoto S, Checler F, Schmitt-Ulms G, St George-Hyslop P, Fraser PE. Pardossi-Piquard R, et al. J Biol Chem. 2009 Oct 16;284(42):28634-41. doi: 10.1074/jbc.M109.059345. Epub 2009 Aug 25. J Biol Chem. 2009. PMID: 19710022 Free PMC article. - NEAT1 and paraspeckles in neurodegenerative diseases: A missing lnc found?
An H, Williams NG, Shelkovnikova TA. An H, et al. Noncoding RNA Res. 2018 Nov 15;3(4):243-252. doi: 10.1016/j.ncrna.2018.11.003. eCollection 2018 Dec. Noncoding RNA Res. 2018. PMID: 30533572 Free PMC article. Review.
References
- Alves da Costa C, Mattson M, Ancolio K, Checler F (2003). The C-terminal fragment of presenilin 2 triggers p53-mediated staurosporine-induced apoptosis, a function independent of the presenilinase-derived N-terminal counterpart. J Biol Chem 278:12064–12069. - PubMed
- Araki W, Yuasa K, Takeda S, Shirotani K, Takahashi K, Tabira T (2000). Overexpression of presenilin-2 enhances apoptotic death of cultured cortical neurons. Ann NY Acad Sci 920:241–244. - PubMed
- Araki W, Yuasa K, Takeda S, Takeda K, Shirotani K, Takahashi K, Tabira T (2001). Pro-apoptotic effect of presenilin 2 (PS2) overexpression is associated with down-regulation of Bcl-2 in cultured neurons. J Neurochem 79:1161–1168. - PubMed
- Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG (2002). Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-κB and β-amyloid precursor protein. Cell 110:55–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous