FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal - PubMed (original) (raw)
FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal
Han You et al. J Exp Med. 2006.
Erratum in
- Correction: FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal.
You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. You H, et al. J Exp Med. 2021 Feb 1;218(2):jem.2006035301132021c. doi: 10.1084/jem.2006035301132021c. J Exp Med. 2021. PMID: 33471042 Free PMC article. No abstract available.
Abstract
Puma is an essential mediator of p53-dependent and -independent apoptosis in vivo. In response to genotoxic stress, Puma is induced in a p53-dependent manner. However, the transcription factor driving Puma up-regulation in response to p53-independent apoptotic stimuli has yet to be identified. Here, we show that FOXO3a up-regulates Puma expression in response to cytokine or growth factor deprivation. Importantly, dysregulated Akt signaling in lymphoid cells attenuated Puma induction upon cytokine withdrawal. Our results suggest that Puma, together with another BH3 only member, Bim, function as FOXO3a downstream targets to mediate a stress response when PI3K/Akt signaling is down-regulated.
Figures
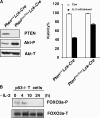
Figure 1.
PI3K/Akt/FOXO3a are involved in cytokine withdrawal-induced apoptosis in activated T cells. (A) Resistance of Pten-deficient activated T cells to apoptosis induced by IL-2 withdrawal. (left) Western blot showing levels of indicated proteins in untreated WT and Pten-deficient T cells. (right) Activated T cells were cultured in IL-2–free medium for 24 h and numbers of viable cells were determined by flow cytometric analysis. Data shown are means ± SD from three independent experiments. (B) FOXO3a phosphorylation status in IL-2–deprived activated T cells derived from p53−/− mice. Phosphorylated FOXO3a (Thr 32) (FOXO3a-P) and total FOXO3a (FOXO3a-T) were detected by Western blotting.
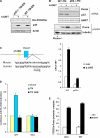
Figure 2.
Puma is a FOXO3a transcriptional target gene. (A) p53+/+ and p53−/− FOXO3a TM-ER MEFs were exposed to 4-OHT (0.5 μM) for 6 h and lysates were subjected to Western blotting using antibodies directed against the indicated proteins. (B) Induction of Puma expression by FOXO3a-TM-ER. Levels of Puma transcripts or Puma protein in WT TM-ER and p53−/− TM-ER MEFs either left untreated (−) or treated with 4-OHT (+) for 8 h were assessed by RT-PCR or Western blotting (top) and QRT-PCR (bottom). HPRT and TBP, normalization control. Cell extracts from puma−/− MEFs were used as a negative control. QRT-PCR data are means ± SD from four independent experiments. (C) Identification of a conserved FHRE site in the human and mouse Puma promoters. p53BE, p53-binding element. (D) FOXO3a-TM activates a luciferase reporter gene driven by the Puma promoter. p53−/− MEFs were cotransfected with constructs as indicated. Luciferase assays were performed 24 h after transfection. Data shown are means ± SD from five independent experiments conducted in triplicates each time. (E) Quantification of FOXO3a association with the Puma promoter. QRT-PCR assays were conducted after chromatin IP using samples from cells that were either left untreated (con) or treated with 4-OHT. Numbers on the y-axis represent the levels of FOXO3a association with the Puma promoter region after normalizing to Ct values from input samples. Data shown are means ± SD from three independent experiments.
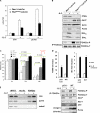
Figure 3.
FOXO3a-dependent regulation of Puma expression in lymphoid cells upon removing of IL-2. (A) Pten loss impairs Puma and bim up-regulation induced by IL-2 deprivation. RNA extracted from Pten +/+ Lck-Cre or Pten flox/flox Lck-Cre activated T cells that were either left untreated or deprived of IL-2 for 10 h was subjected to QRT-PCR. Data represent the mean and error of four independent experiments. (B) Induction of Puma and Bim protein levels was attenuated in the absence of Pten. Activated T cells were deprived of IL-2 for 24 h and cell lysates were subjected to Western blotting with antibodies as indicated. Phosphorylated FOXO3a and total FOXO3a levels were determined by immunoprecipitation and Western blotting. (C) Synergistic cooperation between Puma and Bim in mediating cytokine withdrawal-induced cell death in lymphocytes. T cells were isolated from WT, puma −/−, bim −/−, or DKO mice and expanded in the presence of IL-2 and mitogen. Activated T cells were deprived of IL-2 for the indicated times, cell viability was determined by PI staining, and FACS analysis. p-values (Student's t test) were determined by comparing indicated KO T cells to WT T cells (green line) or DKO T cells to bim −/− T cells (red line). Data shown are means ± SD from three independent experiments. (D) Induction of Puma and bim mRNA (top) and protein levels (bottom) in activated T cells. p53+/+ and p53−/− activated T cells were subjected to IL-2 withdrawal for 10 h. QRT-PCR was performed to detect Puma (top left) and bim (top right) expression levels. Fold induction was determined after normalization to TBP. Results shown are representative of three independent experiments conducted in duplicates each time. (bottom) phosphorylated FOXO3a (Thr 32) (FOXO3a-P) and total FOXO3a (FOXO3a-T) were determined by immunoprecipitation followed by Western blotting. Puma, p53, phosphorylated Akt (Ser473) (Akt-P), and total Akt (Akt-T) were detected by Western blotting. (E) FOXO3a binds to the Puma and bim promoters. Activated T cells generated from p53−/− mice were cultured in IL-2–free medium for 8 h and ChIP assays were conducted as described in Materials and methods.
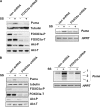
Figure 4.
Direct role of FOXO3a in transcriptional regulating Puma. (A and B) FOXO3a is required for induction of Puma upon serum withdrawal. (left) Western blots show that FOXO3a shRNA successfully blocked FOXO3a expression in p53QSA135V cells (A) or 293T cells (B). Protein levels of Puma, Akt-T/P, and tubulin before and after serum starvation (24 h) were detected by Western blotting. (right) RT-PCR shows that Puma transcripts were elevated in serum-starved p53QSA135V cells (A) or 293T cells (B) expressing control shRNA (con). Ablation of endogenous FOXO3a by FOXO3a shRNA inhibited this elevation in Puma expression in both cell types. SS, serum starvation.
Similar articles
- Inhibition of AKT/FoxO3a signaling induced PUMA expression in response to p53-independent cytotoxic effects of H1: A derivative of tetrandrine.
Zhang YX, Liu XM, Wang J, Li J, Liu Y, Zhang H, Yu XW, Wei N. Zhang YX, et al. Cancer Biol Ther. 2015;16(6):965-75. doi: 10.1080/15384047.2015.1040950. Epub 2015 Apr 20. Cancer Biol Ther. 2015. PMID: 25893985 Free PMC article. - The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells.
Ekoff M, Kaufmann T, Engström M, Motoyama N, Villunger A, Jönsson JI, Strasser A, Nilsson G. Ekoff M, et al. Blood. 2007 Nov 1;110(9):3209-17. doi: 10.1182/blood-2007-02-073957. Epub 2007 Jul 18. Blood. 2007. PMID: 17634411 Free PMC article. - CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress.
Ghosh AP, Klocke BJ, Ballestas ME, Roth KA. Ghosh AP, et al. PLoS One. 2012;7(6):e39586. doi: 10.1371/journal.pone.0039586. Epub 2012 Jun 28. PLoS One. 2012. PMID: 22761832 Free PMC article. - "Sly as a FOXO": new paths with Forkhead signaling in the brain.
Maiese K, Chong ZZ, Shang YC. Maiese K, et al. Curr Neurovasc Res. 2007 Nov;4(4):295-302. doi: 10.2174/156720207782446306. Curr Neurovasc Res. 2007. PMID: 18045156 Free PMC article. Review. - Post-transcriptional regulation of cytokine signaling by AU-rich and GU-rich elements.
Vlasova-St Louis I, Bohjanen PR. Vlasova-St Louis I, et al. J Interferon Cytokine Res. 2014 Apr;34(4):233-41. doi: 10.1089/jir.2013.0108. J Interferon Cytokine Res. 2014. PMID: 24697201 Free PMC article. Review.
Cited by
- Inhibition of AKT/FoxO3a signaling induced PUMA expression in response to p53-independent cytotoxic effects of H1: A derivative of tetrandrine.
Zhang YX, Liu XM, Wang J, Li J, Liu Y, Zhang H, Yu XW, Wei N. Zhang YX, et al. Cancer Biol Ther. 2015;16(6):965-75. doi: 10.1080/15384047.2015.1040950. Epub 2015 Apr 20. Cancer Biol Ther. 2015. PMID: 25893985 Free PMC article. - PTEN: Tumor Suppressor and Metabolic Regulator.
Chen CY, Chen J, He L, Stiles BL. Chen CY, et al. Front Endocrinol (Lausanne). 2018 Jul 9;9:338. doi: 10.3389/fendo.2018.00338. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30038596 Free PMC article. Review. - Resensitization of cisplatin resistance ovarian cancer cells to cisplatin through pretreatment with low-dose fraction radiation.
Zhao L, Liu S, Liang D, Jiang T, Yan X, Zhao S, Liu Y, Zhao W, Yu H. Zhao L, et al. Cancer Med. 2019 May;8(5):2442-2448. doi: 10.1002/cam4.2116. Epub 2019 Apr 2. Cancer Med. 2019. PMID: 30941896 Free PMC article. - The FoxO3a gene is a key negative target of canonical Notch signalling in the keratinocyte UVB response.
Mandinova A, Lefort K, Tommasi di Vignano A, Stonely W, Ostano P, Chiorino G, Iwaki H, Nakanishi J, Dotto GP. Mandinova A, et al. EMBO J. 2008 Apr 23;27(8):1243-54. doi: 10.1038/emboj.2008.45. Epub 2008 Apr 3. EMBO J. 2008. PMID: 18388864 Free PMC article. - Green tea and PUMA: a deadly combination?
Dudgeon C, Yu J. Dudgeon C, et al. Cancer Biol Ther. 2008 Jun;7(6):909-10. doi: 10.4161/cbt.7.6.6317. Epub 2008 May 21. Cancer Biol Ther. 2008. PMID: 18509259 Free PMC article. No abstract available.
References
- Clarke, A.R., C.A. Purdie, D.J. Harrison, R.G. Morris, C.C. Bird, M.L. Hooper, and A.H. Wyllie. 1993. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 362:849–852. - PubMed
- Strasser, A., A.W. Harris, T. Jacks, and S. Cory. 1994. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 79:329–339. - PubMed
- Stahl, M., P.F. Dijkers, G.J. Kops, S.M. Lens, P.J. Coffer, B.M. Burgering, and R.H. Medema. 2002. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 168:5024–5031. - PubMed
- Yu, J., L. Zhang, P.M. Hwang, K.W. Kinzler, and B. Vogelstein. 2001. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell. 7:673–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous