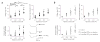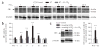Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis - PubMed (original) (raw)
Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis
Sebastian Schuchmann et al. Nat Med. 2006 Jul.
Abstract
Febrile seizures are frequent during early childhood, and prolonged (complex) febrile seizures are associated with an increased susceptibility to temporal lobe epilepsy. The pathophysiological consequences of febrile seizures have been extensively studied in rat pups exposed to hyperthermia. The mechanisms that trigger these seizures are unknown, however. A rise in brain pH is known to enhance neuronal excitability. Here we show that hyperthermia causes respiratory alkalosis in the immature brain, with a threshold of 0.2-0.3 pH units for seizure induction. Suppressing alkalosis with 5% ambient CO2 abolished seizures within 20 s. CO2 also prevented two long-term effects of hyperthermic seizures in the hippocampus: the upregulation of the I(h) current and the upregulation of CB1 receptor expression. The effects of hyperthermia were closely mimicked by intraperitoneal injection of bicarbonate. Our work indicates a mechanism for triggering hyperthermic seizures and suggests new strategies in the research and therapy of fever-related epileptic syndromes.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Figures
Figure 1
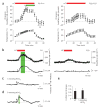
Hyperthermia-induced behavioral seizures are associated with brain alkalosis. (a) Hyperthermia (HT)-induced changes in body temperature (lower panels) and breathing rate (upper panels) in P8–P11 (n = 21; left panels) and P22–P23 (right panels) rat pups. The green bar marks the onset and end of the hyperthermia-induced seizures; the red bar indicates exposure to hyperthermia. Data here and below are mean ± (or +) s.d. Seizures did not occur in P22–P23 rats. (b) Simultaneous recording of hyperthermia-induced changes in breathing rate and intracortical pH in a P9 (left) and a P23 (right) rat. The green bar indicates hyperthermia-induced seizures. (c) Intraperitoneal application (arrow) of 1 mmol/kg bicarbonate induces a small (~0.1 pH units) alkaline shift in pH in the brain of a P9 rat, but no seizure activity. (d) Application of 5 mmol/kg bicarbonate in a P9 rat induces an intracortical alkalosis of 0.24 pH units. The green bar indicates seizure activity. (e) Summary of cortical pH changes in P8–P11 rat pups at threshold for seizure initiation during hyperthermia (n = 9) and upon injection of 5 mmol/kg bicarbonate (n = 7). The two sets of data are not statistically different (P = 0.424).
Figure 2
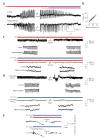
Exposure of rat pups to 5% ambient CO2 blocks hyperthermia- and bicarbonate-induced brain alkalosis and associated ictal activity. (a) Recording in a P9 rat showing simultaneous hyperthermia (HT)-induced ictal activity in the hippocampus and in the temporal cortex, and the fast antiepileptic action of ambient 5% CO2. (b) Time delay between exposure of the pups to 5% CO2 to the block of ongoing ictal activity in the cortex and hippocampus in the P8–P11 rats (n = 29). (c) Cortical recording during a continuous 25-min hyperthermia-induced seizure (upper panel) in a P9 rat. Under identical hyperthermic conditions, but in the presence of 5% CO2, no ictal activity is observed (lower panel). The insets show simultaneous cortical and hippocampal recordings at an expanded scale. (d) Cortical recording immediately after the onset and before the end of ictal activity evoked by intraperitoneal application of 5 mmol/kg bicarbonate (upper panel) in a P10 rat. The bicarbonate-induced ictal activity is completely suppressed by exposure to 5% CO2 (lower panel). The insets show simultaneous cortical and hippocampal recordings at an expanded scale. (e) The alkalosis induced by hyperthermia or intraperitoneal injection of 5 mmol/kg bicarbonate (P9 rats) is completely abolished in the presence of 5% CO2. During prolonged exposure to 5% CO2, a slow acid shift is seen.
Figure 3
Ambient 5% CO2 applied during hyperthermia blocks the long-term upregulation of the I_h_ current. (a) The amplitudes of the I_h_-generated voltage sag and rebound depolarization evoked by hyperpolarizing current pulses in CA1 pyramidal neurons in hippocampal slices are enhanced in slices from pups previously exposed to hyperthermia compared to control slices, but there are no significant differences between neurons from control and hyperthermia + 5% CO2 rats (control,n (slices) = 6; hyperthermia,n = 7; hyperthermia + 5% CO2, n = 5). The inset shows recordings performed 10 d after the induction of hyperthermia-induced seizures at P9. The number of action potentials evoked by the rebound depolarization after hyperpolarizing currents ≥ 0.3 nA is increased in slices from pups previously exposed to hyperthermia. No significant differences were found between neurons from hyperthermia + 5% CO2 and control rats (bottom left panel; control,n (slices) = 5; hyperthermia,n = 6; hyperthermia + 5% CO2, n = 4). Five to ten current pulses of each amplitude shown on the x-axis were applied. Specimen traces are shown on the bottom right. (b) After seizures caused by one or two bicarbonate injections, the I_h_-generated sag (top left panel), the rebound depolarization (top right panel) and the number of action potentials evoked by the rebound depolarization after hyperpolarizing currents ≥ 0.3 nA (bottom panel) are enhanced (control, n (slices) = 8; one bicarbonate injection, n = 8; two bicarbonate injections, n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 for test versus control (ANOVA).
Figure 4
Ambient 5% CO2 applied during hyperthermia blocks the long-term upregulation of the CB1 receptors. (a) Western blots of CB1 receptor protein from hippocampi 1, 2, 3, 5, 10 and 120 d after exposing P10 rats to hyperthermia (red) or to the hyperthermia + 5% CO2 treatment (blue), and from control littermates (white). β-tubulin was used as a reference protein for the quantification shown in b. (b) Quantitative analysis of western blots. An upregulation of the CB1 receptor protein is evident after 2 d following hyperthermia, and a maximum is seen after 5 d. No significant change in CB1 receptor protein expression was found in the hyperthermia + 5% CO2 rats compared to controls (all values represent percentage changes normalized to β-tubulin levels). The value at each time point is based on data from 12 hippocampi. (c) After bicarbonate injection, an upregulation in CB1 receptor is seen at 5 d after seizures (n = 6). This effect is fully blocked in rats in which the seizures were suppressed by 5% CO2. ***P < 0.001, control versus hyperthermia, or control versus bicarbonate injection.
Comment in
- Putting the heat on febrile seizures.
Tapia R. Tapia R. Nat Med. 2006 Jul;12(7):729-30. doi: 10.1038/nm0706-729. Nat Med. 2006. PMID: 16829913 No abstract available. - Respiratory alkalosis: "basic" mechanism of febrile seizures?
Mazarati AM. Mazarati AM. Epilepsy Curr. 2007 Jan-Feb;7(1):25-7. doi: 10.1111/j.1535-7511.2007.00158.x. Epilepsy Curr. 2007. PMID: 17304348 Free PMC article. No abstract available.
Similar articles
- TRPV1 deletion exacerbates hyperthermic seizures in an age-dependent manner in mice.
Barrett KT, Wilson RJ, Scantlebury MH. Barrett KT, et al. Epilepsy Res. 2016 Dec;128:27-34. doi: 10.1016/j.eplepsyres.2016.10.016. Epub 2016 Oct 25. Epilepsy Res. 2016. PMID: 27810513 - Pronounced increase in breathing rate in the "hair dryer model" of experimental febrile seizures.
Schuchmann S, Tolner EA, Marshall P, Vanhatalo S, Kaila K. Schuchmann S, et al. Epilepsia. 2008 May;49(5):926-8. doi: 10.1111/j.1528-1167.2008.01557.x. Epub 2008 Mar 4. Epilepsia. 2008. PMID: 18325016 - Respiratory alkalosis in children with febrile seizures.
Schuchmann S, Hauck S, Henning S, Grüters-Kieslich A, Vanhatalo S, Schmitz D, Kaila K. Schuchmann S, et al. Epilepsia. 2011 Nov;52(11):1949-55. doi: 10.1111/j.1528-1167.2011.03259.x. Epub 2011 Sep 12. Epilepsia. 2011. PMID: 21910730 - Neurobiological and physiological mechanisms of fever-related epileptiform syndromes.
Schuchmann S, Vanhatalo S, Kaila K. Schuchmann S, et al. Brain Dev. 2009 May;31(5):378-82. doi: 10.1016/j.braindev.2008.11.011. Epub 2009 Feb 7. Brain Dev. 2009. PMID: 19201562 Review. - Febrile seizures: mechanisms and relationship to epilepsy.
Dubé CM, Brewster AL, Baram TZ. Dubé CM, et al. Brain Dev. 2009 May;31(5):366-71. doi: 10.1016/j.braindev.2008.11.010. Epub 2009 Feb 15. Brain Dev. 2009. PMID: 19232478 Free PMC article. Review.
Cited by
- High temperatures alter physiological properties of pyramidal cells and inhibitory interneurons in hippocampus.
Kim JA, Connors BW. Kim JA, et al. Front Cell Neurosci. 2012 Jul 6;6:27. doi: 10.3389/fncel.2012.00027. eCollection 2012. Front Cell Neurosci. 2012. PMID: 22783167 Free PMC article. - Targeting choroid plexus epithelium as a novel therapeutic strategy for hydrocephalus.
Yang Y, He J, Wang Y, Wang C, Tan C, Liao J, Tong L, Xiao G. Yang Y, et al. J Neuroinflammation. 2022 Jun 17;19(1):156. doi: 10.1186/s12974-022-02500-3. J Neuroinflammation. 2022. PMID: 35715859 Free PMC article. Review. - Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy.
Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Oakley JC, et al. Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):3994-9. doi: 10.1073/pnas.0813330106. Epub 2009 Feb 20. Proc Natl Acad Sci U S A. 2009. PMID: 19234123 Free PMC article. - Neonatal Seizure Models to Study Epileptogenesis.
Kasahara Y, Ikegaya Y, Koyama R. Kasahara Y, et al. Front Pharmacol. 2018 Apr 18;9:385. doi: 10.3389/fphar.2018.00385. eCollection 2018. Front Pharmacol. 2018. PMID: 29720941 Free PMC article. Review. - NH4Cl-induced metabolic acidosis increases the abundance of HCO3 - transporters in the choroid plexus of mice.
Johnsen LØ, Sigad A, Friis KA, Berg PM, Damkier HH. Johnsen LØ, et al. Front Physiol. 2024 Oct 21;15:1491793. doi: 10.3389/fphys.2024.1491793. eCollection 2024. Front Physiol. 2024. PMID: 39497701 Free PMC article.
References
- Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(Suppl 2):S1–S6. - PubMed
- Tsuboi T. Epidemiology of febrile and afebrile convulsions in children in Japan. Neurology. 1984;34:175–181. - PubMed
- Sagar HJ, Oxbury JM. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsions. Ann Neurol. 1987;22:334–340. - PubMed
- French JA, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. - PubMed
- Holtzman D, Obana K, Olson J. Hyperthermia-induced seizures in the rat pup: a model for febrile convulsions in children. Science. 1981;213:1034–1036. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical