Abeta42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation - PubMed (original) (raw)
Abeta42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation
Arlene M Manelli et al. Neurobiol Aging. 2007 Aug.
Abstract
Autosomal dominant mutations that increase amyloid-beta(1-42) (Abeta42) cause familial Alzheimer's disease (AD), and the most common genetic risk factor for AD is the presence of the epsilon4 allele of apolipoprotein E (apoE). Previously, we characterized stable preparations of Abeta42 oligomers and fibrils and reported that oligomers induced a 10-fold greater increase in neurotoxicity than fibrils in Neuro-2A cells. To determine the effects of apoE genotype on Abeta42 oligomer- and fibril-induced neurotoxicity in vitro, we co-cultured wild type (WT) neurons with glia from WT, apoE-knockout (apoE-KO), and human apoE2-, E3-, and E4-targeted replacement (TR) mice. Dose-dependent neurotoxicity was induced by oligomeric Abeta42 with a ranking order of apoE4-TR>KO=apoE2-TR=apoE3-TR>WT. Neurotoxicity induced by staurosporine or glutamate were not affected by apoE genotype, indicating specificity for oligomeric Abeta42-induced neurotoxicity. These in vitro data demonstrate a gain of negative function for apoE4, synergistic with oligomeric Abeta42, in mediating neurotoxicity.
Figures
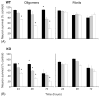
Fig. 1
Oligomeric Aβ42, but not fibrillar Aβ42, induced a dose- and time-dependent increase in neurotoxicity in the presence of WT glia (A) and KO glia (B). Cortical neurons from WT C57BL/6 mice were co-cultured with glial (~95% astrocytes) cells from WT or apoE-KO mice. Aβ42 oligomers or fibrils were added to cultures at 5 μM (■), 10 μM ( ) and 20 μM (□) and incubated for 24, 48, and 72 h. Results are expressed as percent survival of Aβ42-treated cultures with vehicle-treated controls corresponding to 100% survival. Neurotoxicity was assessed using the ATP assay as described in Section 2. *Significant difference between oligomers and fibrils at equivalent dose and time (p < 0.05).
) and 20 μM (□) and incubated for 24, 48, and 72 h. Results are expressed as percent survival of Aβ42-treated cultures with vehicle-treated controls corresponding to 100% survival. Neurotoxicity was assessed using the ATP assay as described in Section 2. *Significant difference between oligomers and fibrils at equivalent dose and time (p < 0.05).
Fig. 2
Oligomeric Aβ42, but not fibrillar Aβ42, induced a dose-dependent increase in neurotoxicity in the presence of apoE2-TR glia (A), apoE3-TR glia (B) and apoE4-TR glia (C). Cortical neurons from WT C57Bl/6 mice were co-cultured with glia from apoE2-, E3-, or E4-TR mice and exposed to 5 μM (■), 10 μM ( ), and 20 μM (□) Aβ42 oligomers or fibrils for 48 h. Results are expressed as percent survival of Aβ42-treated cultures with vehicle-treated controls corresponding to 100% survival. Neurotoxicity was assessed using the ATP assay as described in Section 2. *Significant difference between oligomers and fibrils at equivalent dose (p < 0.05). #Significant difference between E4 and E2 or E3 at equivalent dose (p < 0.05).
), and 20 μM (□) Aβ42 oligomers or fibrils for 48 h. Results are expressed as percent survival of Aβ42-treated cultures with vehicle-treated controls corresponding to 100% survival. Neurotoxicity was assessed using the ATP assay as described in Section 2. *Significant difference between oligomers and fibrils at equivalent dose (p < 0.05). #Significant difference between E4 and E2 or E3 at equivalent dose (p < 0.05).
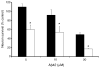
Fig. 3
Aβ42 oligomer-induced neurotoxicity is higher in the absence of glia. WT cortical neurons either alone (□) or in co-culture with WT glia (■) were treated with Aβ42 oligomers (5, 10, or 20 μM) for 48 h. *Significant difference between presence and absence of glia (p < 0.04).
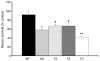
Fig. 4
Neurons co-cultured with apoE4-expressing glia showed the highest oligomeric Aβ42-induced neurotoxicity. Cortical neurons from WT C57BL/6 mice were co-cultured with glia (~95% astrocytes) from WT (■), apoE-KO ( ), apoE2-TR (
), apoE2-TR ( ), apoE3-TR (
), apoE3-TR ( ), or apoE4-TR (□) mice. Oligomeric Aβ42 (10 μM) was added to cultures and incubated for 48 h. Results are expressed as percent survival of Aβ42-treated cultures with vehicle-treated controls corresponding to 100% survival. Neurotoxicity was assessed using the ATP assay as described in Section 2. *Significant difference between WT and apoE-KO (p < 0.04). **Significant difference between apoE-KO and apoE4 (p < 0.04). #Significant difference between E4 and E2 or E3 (p < 0.05).
), or apoE4-TR (□) mice. Oligomeric Aβ42 (10 μM) was added to cultures and incubated for 48 h. Results are expressed as percent survival of Aβ42-treated cultures with vehicle-treated controls corresponding to 100% survival. Neurotoxicity was assessed using the ATP assay as described in Section 2. *Significant difference between WT and apoE-KO (p < 0.04). **Significant difference between apoE-KO and apoE4 (p < 0.04). #Significant difference between E4 and E2 or E3 (p < 0.05).
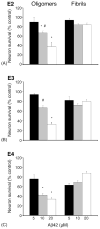
Fig. 5
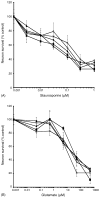
ApoE genotype does not affect glutamate-induced (A) or staurosporine-induced (B) neurotoxicity. Neurotoxicity in WT mouse cortical neurons following 24-h treatment with increasing concentrations of (A) staurosporine, or (B) glutamate was assessed using the ATP assay as described in Section 2. Neurons in co-culture with glia from WT (■), apoE-KO (◆), apoE2-TR (▲), E3-TR (×), or E4-TR (○) mice.
Similar articles
- ApoE protects cortical neurones against neurotoxicity induced by the non-fibrillar C-terminal domain of the amyloid-beta peptide.
Drouet B, Fifre A, Pinçon-Raymond M, Vandekerckhove J, Rosseneu M, Guéant JL, Chambaz J, Pillot T. Drouet B, et al. J Neurochem. 2001 Jan;76(1):117-27. doi: 10.1046/j.1471-4159.2001.00047.x. J Neurochem. 2001. PMID: 11145984 - ApoE isoform-specific effects on LTP: blockade by oligomeric amyloid-beta1-42.
Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Stine WB, Manelli A, Sullivan P, Pasternak JF, LaDu MJ. Trommer BL, et al. Neurobiol Dis. 2005 Feb;18(1):75-82. doi: 10.1016/j.nbd.2004.08.011. Neurobiol Dis. 2005. PMID: 15649697 - ApoE and Abeta1-42 interactions: effects of isoform and conformation on structure and function.
Manelli AM, Stine WB, Van Eldik LJ, LaDu MJ. Manelli AM, et al. J Mol Neurosci. 2004;23(3):235-46. doi: 10.1385/JMN:23:3:235. J Mol Neurosci. 2004. PMID: 15181252 Review. - A dual role for apolipoprotein e in neuroinflammation: anti- and pro-inflammatory activity.
Guo L, LaDu MJ, Van Eldik LJ. Guo L, et al. J Mol Neurosci. 2004;23(3):205-12. doi: 10.1385/JMN:23:3:205. J Mol Neurosci. 2004. PMID: 15181248 - Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease.
Butterfield DA, Mattson MP. Butterfield DA, et al. Neurobiol Dis. 2020 May;138:104795. doi: 10.1016/j.nbd.2020.104795. Epub 2020 Feb 6. Neurobiol Dis. 2020. PMID: 32036033 Free PMC article. Review.
Cited by
- Mercury Ion Binding to Apolipoprotein E Variants ApoE2, ApoE3, and ApoE4: Similar Binding Affinities but Different Structure Induction Effects.
Berntsson E, Sardis M, Noormägi A, Jarvet J, Roos PM, Tõugu V, Gräslund A, Palumaa P, Wärmländer SKTS. Berntsson E, et al. ACS Omega. 2022 Aug 12;7(33):28924-28931. doi: 10.1021/acsomega.2c02254. eCollection 2022 Aug 23. ACS Omega. 2022. PMID: 36033665 Free PMC article. - APOE4 enhances age-dependent decline in cognitive function by down-regulating an NMDA receptor pathway in EFAD-Tg mice.
Liu DS, Pan XD, Zhang J, Shen H, Collins NC, Cole AM, Koster KP, Ben Aissa M, Dai XM, Zhou M, Tai LM, Zhu YG, LaDu M, Chen XC. Liu DS, et al. Mol Neurodegener. 2015 Mar 5;10:7. doi: 10.1186/s13024-015-0002-2. Mol Neurodegener. 2015. PMID: 25871877 Free PMC article. - In vivo and in vitro effects of an apolipoprotein e mimetic peptide on amyloid-β pathology.
Handattu SP, Monroe CE, Nayyar G, Palgunachari MN, Kadish I, van Groen T, Anantharamaiah GM, Garber DW. Handattu SP, et al. J Alzheimers Dis. 2013;36(2):335-47. doi: 10.3233/JAD-122377. J Alzheimers Dis. 2013. PMID: 23603398 Free PMC article. - Endocytic pathways mediating oligomeric Abeta42 neurotoxicity.
Yu C, Nwabuisi-Heath E, Laxton K, Ladu MJ. Yu C, et al. Mol Neurodegener. 2010 May 17;5:19. doi: 10.1186/1750-1326-5-19. Mol Neurodegener. 2010. PMID: 20478062 Free PMC article. - Apolipoprotein E-mediated Modulation of ADAM10 in Alzheimer's Disease.
Shackleton B, Crawford F, Bachmeier C. Shackleton B, et al. Curr Alzheimer Res. 2017;14(6):578-585. doi: 10.2174/1567205014666170203093219. Curr Alzheimer Res. 2017. PMID: 28164773 Free PMC article.
References
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42(9):1681–8. - PubMed
- Banker G, Goslin K. Culturing nerve cells. xiii. Cambridge, MA: MIT Press; 1991. p. 453.
- Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity, implications for neurodegeneration in Alzheimer’s disease brain. A review Free Radic Res. 2002;36(12):1307–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous