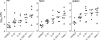Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection - PubMed (original) (raw)
Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection
Bärbel Raupach et al. Infect Immun. 2006 Aug.
Abstract
Caspase-1 (Casp-1) mediates the processing of the proinflammatory cytokines interleukin-1beta (IL-1beta) and IL-18 to their mature forms. Casp-1-deficient mice succumb more rapidly to Salmonella challenge than do wild-type animals. Both Casp-1 substrates, IL-18 and IL-1beta, are relevant for control of Salmonella enterica serovar Typhimurium. We used IL-18-/- and IL-1beta-/- mice in addition to administration of recombinant IL-18 to Casp-1-/- mice to demonstrate that IL-18 is important for resistance to the systemic infection but not for resistance to the intestinal phase of the infection. This suggests that IL-1beta is critical for the intestinal phase of the disease. Thus, we show that Casp-1 is essential for host innate immune defense against S. enterica serovar Typhimurium and that Casp-1 substrates are required at distinct times and anatomical sites.
Figures
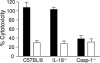
FIG. 1.
Casp-1-deficient macrophages are resistant to S. enterica serovar Typhimurium-induced macrophage death. Macrophages from wild-type, Casp-1-deficient, and IL-18-deficient mice were infected with SL1344 (solid bars) or the noninvasive SPI-1 mutant hilA::mTn5Km2 (open bars). The multiplicity of infection was 100:1. Macrophage death was recorded 3 h postinfection by monitoring the culture supernatants for lactate dehydrogenase activity, using a Cytotox96 cell death kit (Promega). Experiments were performed in triplicate. Means and standard deviations from a representative experiment are shown.
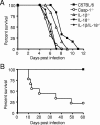
FIG. 2.
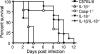
Casp-1-deficient mice rapidly succumb to S. enterica serovar Typhimurium infection. Mice were orally inoculated with S. enterica serovar Typhimurium SL1344, and survival was monitored. (A) Mice with the Nramp1-susceptible C57BL/6 background were challenged with 108 CFU bacteria. Casp-1−/−, IL-1β−/−, IL-18−/−, IL-1β−/−/IL-18−/−, and wild-type mice were used. (B) Mice with the Nramp1-resistant 129Sv/J background were inoculated with 2 × 1010 CFU. ▪, wild-type mice; ○, Casp1−/− mice. Each group contained 9 to 12 mice.
FIG. 3.
Caspase-1-mediated activation of IL-1β and IL-18 increases the susceptibility of Casp-1-deficient mice to oral S. enterica serovar Typhimurium infection. Mice (seven mice per group) were orally inoculated with 5 × 107 CFU S. enterica serovar Typhimurium SL1344, and the bacterial burdens in infected organs were determined on day 5 postinfection. The numbers of bacteria in infected organs were expressed per PP or per g of tissue for MLN and spleens. The bacterial counts for PP, MLN, and spleens of Casp-1−/− mice were significantly higher than the bacterial counts for the organs of wild-type mice (P = 0.0041, P = 0.0012, and P = 0.0111, respectively).
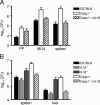
FIG. 4.
Exogenous rIL-18 corrects S. enterica serovar Typhimurium susceptibility in Casp-1−/− mice. Wild-type and gene-deficient animals were infected with 5 × 107 CFU SL1344 orally (A) or with 250 CFU SL1344 intraperitoneally (B). For one group of Casp-1−/− mice, 1.5 μg rIL-18 (Biosource) was injected intraperitoneally daily starting the day prior to Salmonella challenge. The geometric means and standard errors from representative experiments are shown. The number of bacteria in infected organs was expressed per PP or per g of tissue for MLN, spleens, and livers. (A) Bacterial burdens in infected organs (five mice per group) on day 4. The bacterial counts in rIL-18-treated Casp-1−/− mice were significantly reduced in MLN and spleens (P = 0.0357). (B) Bacterial counts in livers and spleens on day 3 postinfection (four mice per group). Pretreatment of Casp-1−/− mice with rIL-18 significantly decreased the bacterial burden (P = 0.0286).
FIG. 5.
IL-18 deficiency protects against septic shock. Mice were injected intraperitoneally with 108 CFU of live attenuated S. enterica serovar Typhimurium SL7207, and survival was monitored. IL-18-proficient mice (C57BL/6 and IL-1β−/− mice) rapidly succumbed to septic shock, while IL-18-deficient animals (Casp-1−/−, IL-18−/−, and IL-1β−/−/IL-18−/− mice) were resistant. Each group contained 9 to 12 mice.
Similar articles
- Gamma interferon-independent effects of interleukin-12 on immunity to Salmonella enterica serovar Typhimurium.
Price JD, Simpfendorfer KR, Mantena RR, Holden J, Heath WR, van Rooijen N, Strugnell RA, Wijburg OL. Price JD, et al. Infect Immun. 2007 Dec;75(12):5753-62. doi: 10.1128/IAI.00971-07. Epub 2007 Sep 17. Infect Immun. 2007. PMID: 17875635 Free PMC article. - Mucosal innate immune response to intragastric infection by Salmonella enterica serovar Choleraesuis.
Hyland KA, Kohrt L, Vulchanova L, Murtaugh MP. Hyland KA, et al. Mol Immunol. 2006 Apr;43(11):1890-9. doi: 10.1016/j.molimm.2005.10.011. Epub 2005 Dec 1. Mol Immunol. 2006. PMID: 16325910 - Enhancement of the anti-Salmonella immune response in CD154-deficient mice by an attenuated, IFN-γ-expressing, strain of Salmonella enterica serovar Typhimurium.
Al-Ojali SM, Moore CB, Fernandez-Cabezudo MJ, Al-Ramadi BK. Al-Ojali SM, et al. Microb Pathog. 2012 Jun;52(6):326-35. doi: 10.1016/j.micpath.2012.03.002. Epub 2012 Mar 13. Microb Pathog. 2012. PMID: 22445817 - Innate immune response to Salmonella typhimurium, a model enteric pathogen.
Broz P, Ohlson MB, Monack DM. Broz P, et al. Gut Microbes. 2012 Mar-Apr;3(2):62-70. doi: 10.4161/gmic.19141. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22198618 Free PMC article. Review. - Lessons from interleukin-deficient mice: the interleukin-1 system.
Fantuzzi G. Fantuzzi G. Acta Physiol Scand. 2001 Sep;173(1):5-9. doi: 10.1046/j.1365-201X.2001.00879.x. Acta Physiol Scand. 2001. PMID: 11678721 Review.
Cited by
- Musca domestica Cecropin (Mdc) Alleviates _Salmonella typhimurium_-Induced Colonic Mucosal Barrier Impairment: Associating With Inflammatory and Oxidative Stress Response, Tight Junction as Well as Intestinal Flora.
Zhang L, Gui S, Liang Z, Liu A, Chen Z, Tang Y, Xiao M, Chu F, Liu W, Jin X, Zhu J, Lu X. Zhang L, et al. Front Microbiol. 2019 Mar 15;10:522. doi: 10.3389/fmicb.2019.00522. eCollection 2019. Front Microbiol. 2019. PMID: 30930887 Free PMC article. - Getting "Inside" Type I IFNs: Type I IFNs in Intracellular Bacterial Infections.
Snyder DT, Hedges JF, Jutila MA. Snyder DT, et al. J Immunol Res. 2017;2017:9361802. doi: 10.1155/2017/9361802. Epub 2017 Apr 26. J Immunol Res. 2017. PMID: 28529959 Free PMC article. Review. - Reassessing the Evolutionary Importance of Inflammasomes.
Maltez VI, Miao EA. Maltez VI, et al. J Immunol. 2016 Feb 1;196(3):956-62. doi: 10.4049/jimmunol.1502060. J Immunol. 2016. PMID: 26802061 Free PMC article. Review. - Pyroptosis of Salmonella Typhimurium-infected macrophages was suppressed and elimination of intracellular bacteria from macrophages was promoted by blocking QseC.
Li Z, Zheng Q, Xue X, Shi X, Zhou Y, Da F, Qu D, Hou Z, Luo X. Li Z, et al. Sci Rep. 2016 Nov 17;6:37447. doi: 10.1038/srep37447. Sci Rep. 2016. PMID: 27853287 Free PMC article. - A role for IL-18 in protective immunity against Mycobacterium tuberculosis.
Schneider BE, Korbel D, Hagens K, Koch M, Raupach B, Enders J, Kaufmann SH, Mittrücker HW, Schaible UE. Schneider BE, et al. Eur J Immunol. 2010 Feb;40(2):396-405. doi: 10.1002/eji.200939583. Eur J Immunol. 2010. PMID: 19950174 Free PMC article.
References
- Biet, F., C. Locht, and L. Kremer. 2002. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J. Mol. Med. 80:147-162. - PubMed
- Birmingham, C. L., A. C. Smith, M. A. Bakowski, T. Yoshimori, and J. H. Brumell. 2006. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281:11374-11383. - PubMed
- Chen, L. M., K. Kaniga, and J. E. Galan. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21:1101-1115. - PubMed
- Dinarello, C. A. 2003. Anti-cytokine therapeutics and infections. Vaccine 21(Suppl. 2):S24-S34. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous