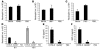MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals - PubMed (original) (raw)
MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals
Chun-Jen Chen et al. J Clin Invest. 2006 Aug.
Abstract
While it is known that monosodium urate (MSU) crystals cause the disease gout, the mechanism by which these crystals stimulate this inflammatory condition has not been clear. Here we find that the Toll/IL-1R (TIR) signal transduction adaptor myeloid differentiation primary response protein 88 (MyD88) is required for acute gouty inflammation. In contrast, other TIR adaptor molecules, TIRAP/Mal, TRIF, and TRAM, are not required for this process. The MyD88-dependent TLR1, -2, -4, -6, -7, -9, and -11 and IL-18 receptor (IL-18R) are not essential for MSU-induced inflammation. Moreover, MSU does not stimulate HEK cells expressing TLR1-11 to activate NF-kappaB. In contrast, mice deficient in the MyD88-dependent IL-1R showed reduced inflammatory responses, similar to those observed in MyD88-deficient mice. Similarly, mice treated with IL-1 neutralizing antibodies also showed reduced MSU-induced inflammation, demonstrating that IL-1 production and IL-1R activation play essential roles in MSU-triggered inflammation. IL-1R deficiency in bone marrow-derived cells did not affect the inflammatory response; however, it was required in non-bone marrow-derived cells. These results indicate that IL-1 is essential for the MSU-induced inflammatory response and that the requirement of MyD88 in this process is primarily through its function as an adaptor molecule in the IL-1R signaling pathway.
Figures
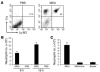
Figure 1. MSU induces peritonitis in mice.
(A and B) C57BL/6 mice were challenged i.p. with 3 mg of MSU crystals. Mice challenged with PBS served as negative controls. Peritoneal cells were stained for Ly-6G and 7/4 expression and analyzed by flow cytometry. (A) Representative dot plots of Ly-6G and 7/4 expression on peritoneal cells in animals injected 6 hours earlier with PBS or MSU. The Ly-6G+7/4+ gate represents neutrophils. (B) At indicated time points, neutrophil numbers in PECs were determined by multiplying the total cell numbers by the percentage of Ly-6G+7/4+ cells (n = 3). (C) C57BL/6 mice were challenged i.p. with 1.5 mg of MSU crystals, 1.5 mg of uricase-digested MSU (MSU+Uox), or borate buffer. At 16 hours after challenge, neutrophil numbers in the PECs were determined (n = 3). Data shown are representative of 3 or more experiments.
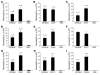
Figure 2. MSU-induced inflammation does not require TLR1, -2, -3, -4, -6, -7, -9, and -11.
C57BL/6 mice and mice deficient for TLR1 (A), TLR2 (B), TLR3 (C), TLR4 (D), TLR6 (E), TLR7 (F), TLR9 (G), TLR11 (H), or TLR2/4 (I) were challenged i.p. with 3 mg of MSU crystals (in PBS). C57BL/6 mice challenged with PBS served as negative controls. At 16 hours after challenge, neutrophil numbers in PECs were determined. Data (mean ± SEM) shown in A–E and G–I were combined from 3 independent experiments, and _n_’s reported represent the combined numbers of animals for each group. Data shown in F are from a single experiment due to the limited availability of animals (n = 3).
Figure 3. MSU does not stimulate transfected TLRs in vitro.
HEK cells were prepared as described in Methods. The following TLR ligands were added to the cells: Malp2 (100 ng/ml), Poly(I:C) (200 μg/ml), LPS (1 ng/ml), R-848 (1 μg/ml), and CpG (5 μg/ml). TLR10 and TLR11 ligands were unavailable for the test. The readout is the fold increase of firefly luciferase/Renilla luciferase ratio over the unstimulated control. Data shown are representative of 2 or more experiments.
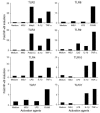
Figure 4. MyD88, but not TRAM, Mal, or TRIF, is required for MSU-induced KC and MIP-2 production and neutrophil infiltration in the peritoneal cavity.
(A–C) B6129 mice and mice deficient for TRAM (A), Mal (B), or TRIF (C) were challenged i.p. with 3 mg of MSU crystals (in PBS). B6129 mice challenged with PBS served as negative controls. At 16 hours after MSU challenge, neutrophil numbers in the PECs were determined (n = 3). (D–F) C57BL/6 and MyD88–/– mice were challenged i.p. with 3 mg of MSU crystals (in PBS). C57BL/6 mice challenged with PBS served as negative controls. (D) At the indicated time points, neutrophil numbers in the PECs were determined (n = 3 for 6 hours and n = 4 for 16 hours). At 6 hours after MSU challenge, KC (E) and MIP-2 (F) concentrations in the peritoneal lavage fluids were determined by ELISA (n = 3). Data shown are representative of 3 or more experiments. *P < 0.01 versus control (C57BL/6) in D–F.
Figure 5. MSU-induced inflammation is IL-1R dependent and IL-18R independent.
C57BL/6 mice and mice deficient for IL-1R (A–C) or IL-18R (D) were challenged i.p. with 3 mg of MSU crystals (in PBS). C57BL/6 mice challenged with PBS served as negative controls. (A) At indicated time points, neutrophil numbers in the PECs were determined (n = 4). At 6 hours after MSU challenge, KC (B) and MIP-2 (C) concentrations in the peritoneal lavage fluids were determined by ELISA (n = 4). (D) At 16 hours after MSU challenge, neutrophil numbers in the PECs were determined (n = 4). Data shown in A–C are representative of 3 or more experiments. Data shown in D are representative of 2 experiments. *P < 0.01 versus control (C57BL/6) in A and B.
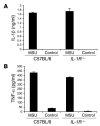
Figure 6. IL-1R is not the receptor for MSU stimulation.
Peritoneal resident cells from C57BL/6 and IL-1R–/– mice were harvested and stimulated with or without MSU crystals as described in Methods. At 16 hours after MSU stimulation, IL-1β (A) and TNF-α (B) concentrations in the culture fluids were determined by ELISA (n = 2). Data shown are representative of 3 or more experiments.
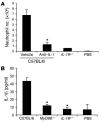
Figure 7. The role of IL-1 in MSU-induced inflammation.
(A) C57BL/6 mice were administered i.v. with anti–IL-1 antibodies or PBS (vehicle). Thirty minutes later, the treated mice and IL-1R–/– mice were challenged i.p. with MSU crystals. C57BL/6 mice challenged with PBS served as negative controls. At 6 hours after MSU challenge, neutrophil numbers in the PECs were determined (n = 4). *P < 0.01 versus control (vehicle). (B) C57BL/6, MyD88–/–, and IL-1R–/– mice were challenged i.p. with 3 mg of MSU crystals (in PBS). C57BL/6 mice challenged with PBS served as negative controls. At 6 hours after MSU challenge, IL-1β concentration in the peritoneal lavage fluids were determined by ELISA (n = 5). Data shown are representative of 2 experiments in A and 3 experiments in B. *P < 0.01 versus control (C57BL/6).
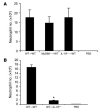
Figure 8. Functions of IL-1R and MyD88 in bone marrow– versus non–bone marrow–derived cells mediating MSU-stimulated inflammation.
Bone marrow chimeras were generated as described in Methods. (A) C57BL/6 (WT) mice served as hosts, and B6.SJL (WT), MyD88–/–, or IL-1R–/– mice served as bone marrow donors. (B) C57BL/6 (WT) and IL-1R–/– mice served as hosts, and B6.SJL (WT) mice served as bone marrow donors. (A and B) After bone marrow reconstitution, chimeric mice were challenged i.p. with 3 mg of MSU crystals (in PBS). C57BL/6 mice challenged with PBS served as negative controls. At 16 hours after MSU challenge, neutrophil numbers in the PECs were determined (n = 3). Data shown in A are representative of 8 experiments; data shown in B are representative of 3 experiments. *P < 0.01 versus control (WT→WT).
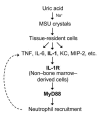
Figure 9. Proposed mechanism for MSU-induced inflammation.
When uric acid nucleates to form MSU crystals, tissue resident cells are stimulated to produce IL-1 and other cytokines. The IL-1 that is produced then stimulates the IL-1R on non–bone marrow–derived cells, resulting in a MyD88-dependent amplification of the proinflammatory response.
Comment in
- Gout: new insights into an old disease.
Martinon F, Glimcher LH. Martinon F, et al. J Clin Invest. 2006 Aug;116(8):2073-5. doi: 10.1172/JCI29404. J Clin Invest. 2006. PMID: 16886051 Free PMC article.
Similar articles
- Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation.
Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Liu-Bryan R, et al. Arthritis Rheum. 2005 Sep;52(9):2936-46. doi: 10.1002/art.21238. Arthritis Rheum. 2005. PMID: 16142712 - Gout: new insights into an old disease.
Martinon F, Glimcher LH. Martinon F, et al. J Clin Invest. 2006 Aug;116(8):2073-5. doi: 10.1172/JCI29404. J Clin Invest. 2006. PMID: 16886051 Free PMC article. - A unique feature of Toll/IL-1 receptor domain-containing adaptor protein is partially responsible for lipopolysaccharide insensitivity in zebrafish with a highly conserved function of MyD88.
Liu Y, Li M, Fan S, Lin Y, Lin B, Luo F, Zhang C, Chen S, Li Y, Xu A. Liu Y, et al. J Immunol. 2010 Sep 15;185(6):3391-400. doi: 10.4049/jimmunol.0903147. Epub 2010 Aug 11. J Immunol. 2010. PMID: 20702732 - [Pathological mechanisms of gouty arthritis].
Akahoshi T. Akahoshi T. Nihon Rinsho. 2008 Apr;66(4):705-10. Nihon Rinsho. 2008. PMID: 18409519 Review. Japanese. - Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways.
Narayanan KB, Park HH. Narayanan KB, et al. Apoptosis. 2015 Feb;20(2):196-209. doi: 10.1007/s10495-014-1073-1. Apoptosis. 2015. PMID: 25563856 Review.
Cited by
- The role of HCA2 (GPR109A) in regulating macrophage function.
Zandi-Nejad K, Takakura A, Jurewicz M, Chandraker AK, Offermanns S, Mount D, Abdi R. Zandi-Nejad K, et al. FASEB J. 2013 Nov;27(11):4366-74. doi: 10.1096/fj.12-223933. Epub 2013 Jul 23. FASEB J. 2013. PMID: 23882124 Free PMC article. - Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology.
Greenhalgh AD, Brough D, Robinson EM, Girard S, Rothwell NJ, Allan SM. Greenhalgh AD, et al. Dis Model Mech. 2012 Nov;5(6):823-33. doi: 10.1242/dmm.008557. Epub 2012 May 31. Dis Model Mech. 2012. PMID: 22679224 Free PMC article. - Identification of Toll-like receptor signaling inhibitors based on selective activation of hierarchically acting signaling proteins.
Ippagunta SK, Pollock JA, Sharma N, Lin W, Chen T, Tawaratsumida K, High AA, Min J, Chen Y, Guy RK, Redecke V, Katzenellenbogen JA, Häcker H. Ippagunta SK, et al. Sci Signal. 2018 Aug 14;11(543):eaaq1077. doi: 10.1126/scisignal.aaq1077. Sci Signal. 2018. PMID: 30108181 Free PMC article. - Update on biology: uric acid and the activation of immune and inflammatory cells.
Martinon F. Martinon F. Curr Rheumatol Rep. 2010 Apr;12(2):135-41. doi: 10.1007/s11926-010-0092-3. Curr Rheumatol Rep. 2010. PMID: 20425023 Review. - Suppression of Syk activation by resveratrol inhibits MSU crystal-induced inflammation in human monocytes.
Chung YH, Kim HY, Yoon BR, Kang YJ, Lee WW. Chung YH, et al. J Mol Med (Berl). 2019 Mar;97(3):369-383. doi: 10.1007/s00109-018-01736-y. Epub 2019 Jan 12. J Mol Med (Berl). 2019. PMID: 30637441
References
- Terkeltaub, R. 2005. Pathogenesis and treatment of crystal-induced inflammation. InArthritis and allied conditions. W. Koopman and L.W. Moreland, editors. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 2357.
- [Anonymous]. . Carl Wilhelm Scheele (1742-1786) Swedish apothecary. Jama. 1970;212:2258–2259. - PubMed
- Freudweiler M. Studies on the nature of gouty tophi. Dtsch. Arch. Klin. Med. 1899;63:266.
- Rich A.M., Giedd K.N., Cristello P., Weissmann G. Granules are necessary for death of neutrophils after phagocytosis of crystalline monosodium urate. Inflammation. 1985;9:221–232. - PubMed
- Tramontini N., Huber C., Liu-Bryan R., Terkeltaub R.A., Kilgore K.S. Central role of complement membrane attack complex in monosodium urate crystal-induced neutrophilic rabbit knee synovitis. Arthritis Rheum. 2004;50:2633–2639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous