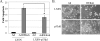Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions - PubMed (original) (raw)
Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions
Trisha M Wise-Draper et al. Mol Cell Biol. 2006 Oct.
Abstract
The DEK proto-oncogene has been associated with human carcinogenesis-either as a fusion with the CAN nucleoporin protein or when transcriptionally upregulated. Mechanisms of intracellular DEK functions, however, have remained relatively unexplored. We have recently demonstrated that DEK expression is induced by the high-risk human papillomavirus (HPV) E7 protein in a manner which is dependent upon retinoblastoma protein function and have implicated DEK in the inhibition of cellular senescence. Additionally, overexpression of DEK resulted in significant life span extension of primary human keratinocytes. In order to determine whether DEK expression is required for cellular proliferation and/or survival, we monitored cellular responses to the knockdown of DEK in cancer and primary cells. The results indicate that DEK expression protects both HPV-positive cancer and primary human cells from apoptotic cell death. Cell death in response to DEK depletion was accompanied by increased protein stability and transcriptional activity of the p53 tumor suppressor and consequent upregulation of known p53 target genes such as p21CIP and Bax. Consistent with a possible role for p53 in DEK-mediated cell death inhibition, the p53-negative human osteosarcoma cell line SAOS-2 was resistant to the knockdown of DEK. Finally, expression of a dominant negative p53 miniprotein inhibited DEK RNA interference-induced p53 transcriptional induction, as well as cell death, thus directly implicating p53 activation in the observed apoptotic phenotype. These findings suggest a novel role for DEK in cellular survival, involving the destabilization of p53 in a manner which is likely to contribute to human carcinogenesis.
Figures
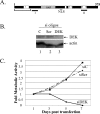
FIG. 1.
DEK depletion represses HeLa cell growth. (A) Schematic representation of the human DEK protein. The conserved SAP DNA binding domain is indicated. A separate C-terminal DNA binding domain has been mapped to aa 270 to 350 (54). Several highly acidic domains containing predominantly glutamic acid are shown as white boxes, and black rectangles represent in vitro casein kinase 2 phosphorylation sites (5). Acetylation within the N-terminal 70 aa is indicated by a bracket. Region I represents the sequence targeted by double-stranded siRNA oligonucleotide delivery, and region II represents the sequence targeted by delivery of the BS/U6-DEKsh plasmid. (B) Western blot analyses. HeLa cells were transfected either with unrelated or scrambled DEK siRNA oligonucleotides (si oligos) as controls (lanes 1 and 2) or with DEK-specific siRNA (lane 3). Whole-cell lysates were harvested on day 4 posttransfection and subjected to SDS-polyacrylamide gel electrophoresis and Western blot analyses with monoclonal DEK and actin antibodies. (C) Cytotoxicity assay. HeLa cells were transfected as in panel B, split, and exposed to MTS reagent for colorimetric detection of cellular metabolic activity on days 1, 3, 5, and 7.
FIG. 2.
Cellular growth arrest following DEK depletion is due to apoptosis. (A) Northern blot analyses. HeLa cells were transfected with either an empty vector (lane 1) or the DEKsh vector (lane 2), and RNA was harvested after 2 days. Equal amounts of RNA were subjected to Northern blot analyses with a full-length cDNA DEK-specific probe. The blot was then stripped and rehybridized with a GAPDH-specific probe. (B) Western blot analyses. Cells were cotransfected with a puromycin resistance plasmid and either an empty vector, a GFP-specific vector, or the DEK-specific short hairpin RNA expression vector. Cells were selected in puromycin, and protein lysates were harvested on days 1 to 5. Equal amounts of protein were subjected to Western blot analyses with DEK- and actin-specific monoclonal antibodies as described in the legend to Fig. 1. (C) Apoptosis assays. HeLa cells were transfected as for panel A and collected on days 1 to 5 for fixation and binding of anti-activated caspase 3 antibody conjugated to fluorescein isothiocyanate. Apoptotic cells were detected and quantitated by flow cytometry. (D) Colony reduction assays. HeLa cells were transfected with a neomycin resistance plasmid and either an empty vector, a DEKsh vector, or a DEKsh vector plus a DEK68-226 expression plasmid. The cells were selected in G418 for 2 weeks, and colonies were stained with methylene blue.
FIG. 3.
DEK depletion does not result in aberrant cell cycle progression. (A) Western blot analyses. HeLa cells were infected with either empty Ad or AdDEKsh at an MOI of 3, 10, or 30 PFU. Total protein was harvested after 4 days and subjected to Western blot analysis as described in Materials and Methods. (B) Transient cell growth assay. HeLa cells were infected with the indicated viruses at an MOI of 100 IUs, and viable cells were counted for 7 days by trypan blue exclusion. (C) Cell cycle analysis. HeLa cells were infected as for panel B and harvested for cell cycle analysis by flow cytometry on days 1 to 4. (D) Apoptosis analysis. HeLa cells were infected as for panel B and harvested for apoptosis analysis by 7-AAD incorporation on days 1 to 4 postinfection. The percentage of apoptosis was determined by quantitation of the sub-G1 population for each sample.
FIG. 4.
DEK overexpression inhibits HeLa cell apoptosis. (A) Transient cell growth assay. HeLa cells were infected with an MOI of 100 IUs of the indicated viruses and counted on each day for a total of 7 days following trypan blue exclusion. (B) Colony assays and Western blot analyses. HeLa cells were infected as described for panel A, and cell density and GFP positivity were verified by bright-field (top) and fluorescence (bottom) microscopy. Sparsely seeded cells were allowed to form colonies over a period of 2 weeks and stained with methylene blue. HeLa cells were infected with either empty Ad or AdDEK at 100 IUs, and total protein was harvested after 7 days and subjected to Western blot analysis as described above. (C) Cell cycle analysis. HeLa cells were Ad or AdDEK infected as described above and fixed on the indicated days postinfection for propidium iodide staining and flow cytometry. Percent cells in the indicated phase of the cell cycle are displayed. (D) Apoptosis assay. HeLa cells were infected as described above and subjected to anti-active caspase 3 biotinylated antibody and streptavidin conjugated to APC detection by flow cytometry on the indicated days postinfection. The percentage of apoptotic cells in each infected-cell population is indicated in the graph. Stars indicate statistical significance with P values of 0.05 for day 7 and 0.02 for day 8.
FIG. 5.
SAOS-2 cells are resistant to cell death in response to DEK depletion. (A) Western blot analyses. SAOS-2 cells were infected with either empty Ad, AdDEK, or AdDEKsh (lanes 1 to 3) at an MOI of 50 IUs. Total protein extracts were harvested after 4 days and subjected to DEK-specific Western blot analyses. (B) Cytotoxicity assays. HeLa and SAOS-2 cells were split into a 96-well plate, infected as described for panel A, and subjected to MTS assays at the indicated time points as described in the legend to Fig. 1. (C) Apoptosis assay. HeLa and SAOS-2 cells were infected as described above, collected on day 5 postinfection, and fixed for flow cytometry as described in the legend to Fig. 4.
FIG. 6.
DEK depletion results in apoptosis in primary human keratinocytes. (A) Western blot analyses. HFKs were infected at an MOI of 100 IUs with the indicated viruses and subjected to DEK-specific Western blot analysis on day 4 postinfection. (B) Transient cell growth assays. HFKs were infected as for panel A, and cells were counted every other day for 6 days by trypan blue exclusion. (C and D) Apoptosis assays. HFKs were infected as described above, harvested on day 5 (C) or day 7 (D) postinfection, and subjected to anti-active caspase 3 antibody and flow cytometry. The graph represents changes in the number of apoptotic cells relative to the empty Ad control, and the stars represent statistical significance with P values of less than 0.01 compared to the empty Ad control.
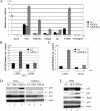
FIG. 7.
DEK inhibits p53 transcriptional activity and the expression of several p53 target genes. Luciferase (luc.) reporter assays. (A) HeLa cells were transfected with the indicated p53-responsive promoter luciferase constructs as described in Materials and Methods and superinfected with Ad, AdDEK, or AdDEKsh. Luciferase extracts were harvested on day 3 postinfection, and luciferase activity was quantitated with a luminometer. The values below the bars indicate changes relative to the empty-Ad-infected cells. (B) HeLa cells were stably transduced with either empty LXSN or the dominant negative p53-expressing retrovirus vector LSXN-p53dd. The respective cell pools were then transfected with the PTGFβ luciferase construct, superinfected with Ad versus AdE2ts at an MOI of 100 IUs, and shifted to the permissive temperature. Expression of the tsE2 protein is known to result in suppression of viral E6/E7 oncogene expression and thus upregulated p53 levels in HeLa cells. Luciferase activities were quantitated as described above. E2-mediated p53 induction activated the PTGFβ reporter, and the observed activation was inhibited by dominant negative p53, supporting the functionality of the p53dd retrovirus construct. (C) Stably transduced LXSN and LSXN-p53dd HeLa cell pools were transfected with the PTGFβ luciferase construct and superinfected on the next day with Ad or AdDEKsh at 100 IUs. Luciferase activities were quantitated as described above. DEK depletion activated the PTGFβ reporter in a manner that was inhibitable by dominant negative p53 expression. (D and E) Western blot analyses. HeLa and SAOS-2 cells, as well as primary HFKs, were infected with the indicated viruses. Protein lysates were harvested 4 days later and subjected to Western blot analyses with p53-, p21-, p16-, BAX-, or actin-specific antibodies.
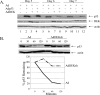
FIG. 8.
DEK modulation affects p53 protein stability. (A) Western blot analyses. Primary HFKs were infected with empty Ad, AdDEK, and Adp53 expression vectors, either alone or in combination, and harvested on the indicated days. Protein lysates were subjected to Western blot analyses with p53-, DEK-, and actin-specific antibodies. (B) Kinetic analysis of DEK-dependent p53 degradation. HeLa cells were infected with either an empty Ad or an AdDEKsh vector at 50 IUs, and the cells were treated with 50 μg/ml cycloheximide 4 days later. Protein extracts were collected at the indicated time points and subjected to Western blot analyses with p53- or actin-specific antibodies. The resulting p53 signals were quantitated with NIH Image and graphed as percent p53 remaining compared to time zero.
FIG. 9.
Apoptosis in response to DEK depletion requires p53. (A) Apoptosis assay. Primary HFKs were stably transduced with either LXSN or dominant negative p53dd retrovirus and then superinfected with either empty Ad or AdDEKsh. Apoptosis was quantitated with the anti-active caspase 3 antibody and flow cytometry to determine the percentage of apoptotic cells 5 days later. The star indicates statistical significance with a P value of 0.03. (B) Cellular phenotype. Cells were infected as for panel A, and pictures were taken on day 5 postinfection.
Similar articles
- The human DEK proto-oncogene is a senescence inhibitor and an upregulated target of high-risk human papillomavirus E7.
Wise-Draper TM, Allen HV, Thobe MN, Jones EE, Habash KB, Münger K, Wells SI. Wise-Draper TM, et al. J Virol. 2005 Nov;79(22):14309-17. doi: 10.1128/JVI.79.22.14309-14317.2005. J Virol. 2005. PMID: 16254365 Free PMC article. - DEK proto-oncogene expression interferes with the normal epithelial differentiation program.
Wise-Draper TM, Morreale RJ, Morris TA, Mintz-Cole RA, Hoskins EE, Balsitis SJ, Husseinzadeh N, Witte DP, Wikenheiser-Brokamp KA, Lambert PF, Wells SI. Wise-Draper TM, et al. Am J Pathol. 2009 Jan;174(1):71-81. doi: 10.2353/ajpath.2009.080330. Epub 2008 Nov 26. Am J Pathol. 2009. PMID: 19036808 Free PMC article. - Melanoma proliferation and chemoresistance controlled by the DEK oncogene.
Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, Kim DS, Chinnaiyan AM, Markovitz DM, Soengas MS. Khodadoust MS, et al. Cancer Res. 2009 Aug 15;69(16):6405-13. doi: 10.1158/0008-5472.CAN-09-1063. Cancer Res. 2009. PMID: 19679545 Free PMC article. - Progress in studies on the DEK protein and its involvement in cellular apoptosis.
Hua Y, Hu H, Peng X. Hua Y, et al. Sci China C Life Sci. 2009 Jul;52(7):637-42. doi: 10.1007/s11427-009-0088-2. Epub 2009 Jul 30. Sci China C Life Sci. 2009. PMID: 19641868 Review. - The DEK oncoprotein and its emerging roles in gene regulation.
Sandén C, Gullberg U. Sandén C, et al. Leukemia. 2015 Aug;29(8):1632-6. doi: 10.1038/leu.2015.72. Epub 2015 Mar 13. Leukemia. 2015. PMID: 25765544 Review.
Cited by
- Novel molecular mechanisms in Alzheimer's disease: The potential role of DEK in disease pathogenesis.
Greene AN, Solomon MB, Privette Vinnedge LM. Greene AN, et al. Front Aging Neurosci. 2022 Oct 6;14:1018180. doi: 10.3389/fnagi.2022.1018180. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36275000 Free PMC article. Review. - The oncoprotein DEK affects the outcome of PARP1/2 inhibition during mild replication stress.
Ganz M, Vogel C, Czada C, Jörke V, Gwosch EC, Kleiner R, Pierzynska-Mach A, Zanacchi FC, Diaspro A, Kappes F, Bürkle A, Ferrando-May E. Ganz M, et al. PLoS One. 2019 Aug 13;14(8):e0213130. doi: 10.1371/journal.pone.0213130. eCollection 2019. PLoS One. 2019. PMID: 31408463 Free PMC article. - High expression of DEK predicts poor prognosis of gastric adenocarcinoma.
Piao J, Shang Y, Liu S, Piao Y, Cui X, Li Y, Lin Z. Piao J, et al. Diagn Pathol. 2014 Mar 20;9:67. doi: 10.1186/1746-1596-9-67. Diagn Pathol. 2014. PMID: 24650035 Free PMC article. - Meta-analysis of nasopharyngeal carcinoma microarray data explores mechanism of EBV-regulated neoplastic transformation.
Chen X, Liang S, Zheng W, Liao Z, Shang T, Ma W. Chen X, et al. BMC Genomics. 2008 Jul 7;9:322. doi: 10.1186/1471-2164-9-322. BMC Genomics. 2008. PMID: 18605998 Free PMC article. - DEK is a poly(ADP-ribose) acceptor in apoptosis and mediates resistance to genotoxic stress.
Kappes F, Fahrer J, Khodadoust MS, Tabbert A, Strasser C, Mor-Vaknin N, Moreno-Villanueva M, Bürkle A, Markovitz DM, Ferrando-May E. Kappes F, et al. Mol Cell Biol. 2008 May;28(10):3245-57. doi: 10.1128/MCB.01921-07. Epub 2008 Mar 10. Mol Cell Biol. 2008. PMID: 18332104 Free PMC article.
References
- Alexander, K., H. S. Yang, and P. W. Hinds. 2003. pRb inactivation in senescent cells leads to an E2F-dependent apoptosis requiring p73. Mol. Cancer Res. 1:716-728. - PubMed
- Arnaudo, J. P., J. Deibener, and P. Kaminsky. 1998. Antibodies to the DEK protein in Kikuchi's disease. J. Rheumatol. 25:1861-1862. - PubMed
- Beroud, C., and T. Soussi. 2003. The UMD-p53 database: new mutations and analysis tools. Hum. Mutat. 21:176-181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous