Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1 - PubMed (original) (raw)
Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1
Jinhee Song et al. Plant Cell. 2006 Sep.
Abstract
Epsin and related proteins play important roles in various steps of protein trafficking in animal and yeast cells. Many epsin homologs have been identified in plant cells from analysis of genome sequences. However, their roles have not been elucidated. Here, we investigate the expression, localization, and biological role in protein trafficking of an epsin homolog, Arabidopsis thaliana EPSIN1, which is expressed in most tissues we examined. In the cell, one pool of EPSIN1 is associated with actin filaments, producing a network pattern, and a second pool localizes primarily to the Golgi complex with a minor portion to the prevacuolar compartment, producing a punctate staining pattern. Protein pull-down and coimmunoprecipitation experiments reveal that Arabidopsis EPSIN1 interacts with clathrin, VTI11, gamma-adaptin-related protein (gamma-ADR), and vacuolar sorting receptor1 (VSR1). In addition, EPSIN1 colocalizes with clathrin and VTI11. The epsin1 mutant, which has a T-DNA insertion in EPSIN1, displays a defect in the vacuolar trafficking of sporamin:green fluorescent protein (GFP), but not in the secretion of invertase:GFP into the medium. Stably expressed HA:EPSIN1 complements this trafficking defect. Based on these data, we propose that EPSIN1 plays an important role in the vacuolar trafficking of soluble proteins at the trans-Golgi network via its interaction with gamma-ADR, VTI11, VSR1, and clathrin.
Figures
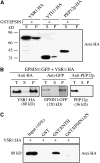
Figure 1.
EPSIN1 Is Expressed in Various Arabidopsis Tissues. (A) Generation of anti-EPSIN1 antibody. The middle domain, corresponding to amino acid residues 153 to 337, was expressed as the Hisx6-tagged form in E. coli and used to raise antibody in a rabbit. Control serum was obtained from the rabbit before immunization. Total protein extracts were obtained from leaf tissues and used to test the anti-EPSIN1 antibody. (B) Specificity of the anti-EPSIN1 antibody. Protein extracts were obtained from protoplasts expressing EPSIN1 tagged with the HA epitope at the N terminus and used for protein gel blot analysis using anti-HA and anti-EPSIN1 antibodies. (C) Expression of EPSIN1 in various tissues. Total protein extracts from the indicated tissues were analyzed by protein gel blotting using anti-EPSIN1 antibody. Leaf tissues were harvested 11 and 20 d after germination. Cotyledons were obtained from 5-d-old plants. The membranes were stained with Coomassie blue to control for protein loading. RbcL, large subunit of the ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco) complex.
Figure 2.
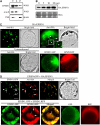
EPSIN1 Produces Both Network and Punctate Staining Patterns. (A) Subcellular fractionation of EPSIN1. Total (T) protein extracts of leaf tissues were separated into soluble (S) and pellet (P) fractions and analyzed by protein gel blotting using anti-EPSIN1, anti-AALP, and anti-VSR antibodies. (B) Expression level of EPSIN1 in transformed protoplasts. Protoplasts were transformed with various amounts of HA:EPSIN1 DNA, and the level of EPSIN1 was determined by protein gel blotting with anti-EPSIN1 antibody. Protein extracts from untransformed protoplasts were used as a control. The membrane was also stained with Coomassie blue to control for loading. (C) Localization of EPSIN1. Protoplasts were transformed with the indicated constructs (5 to 10 μg), and the localization of EPSIN1 was examined either by immunostaining with anti-HA antibody or by direct detection of the GFP or RFP signal. Untransformed protoplasts were immunostained with anti-HA antibody as a control. Bars = 20 μm. (D) Colocalization of EPSIN1 proteins. The localization of EPSIN1 protein was examined in protoplasts transformed with HA:EPSIN1 and EPSIN1:GFP or with EPSIN1:GFP and EPSIN1:RFP. As controls, GFP and RFP alone were transformed into protoplasts. Bars = 20 μm.
Figure 3.
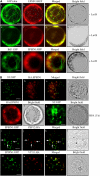
Localization of EPSIN1 in Protoplasts. (A) Colocalization of EPSIN1 with actin filaments. Protoplasts were transformed with the indicated constructs, and the localization of these proteins was examined in the presence (+ Lat B) and absence (− Lat B) of Lat B (10 μM). Bars = 20 μm. (B) Localization of EPSIN1 to the Golgi complex and the PVC. Protoplasts were transformed with the indicated constructs, and localization of the proteins was examined after immunostaining with anti-HA. The GFP signals were observed directly in the fixed protoplasts. For BFA treatment, BFA (30 μg/mL) was added to the transformed protoplasts at 24 h after transformation and incubated for 3 h. Arrows indicate the overlap between EPSIN1:GFP and PEP12p:HA. Bars = 20 μm.
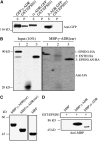
Figure 4.
EPSIN1 Binds to and Colocalizes with Clathrin. (A) Constructs. GST was fused to the N terminus. ENTH, the epsin N-terminal homology domain. DLF and DPF motifs are similar to AP-1 and AP-3 binding motifs, respectively. Q11 indicates a stretch of 11 Glu residues. The clathrin binding motif (LIDL) and the Leu-to-Arg substitution in the clathrin binding motif (RIDL) are shown in the C-terminal region. The numbers indicate amino acid positions. (B) Expression of GST-fused EPSIN1 proteins. Constructs were introduced into E. coli, and their expression was induced by isopropylthio-β-galactoside.GST fusion proteins were purified from E. coli extracts with glutathione–agarose beads. Purified proteins were stained with Coomassie blue. (C) Interaction of EPSIN1 with clathrin. GST-fused EPSIN1 proteins were mixed with protein extracts from leaf tissues. EPSIN1 binding proteins were precipitated using glutathione–agarose beads and analyzed by protein gel blotting using anti-clathrin antibody. Supernatants also were included in the protein gel blot analysis. Subsequently, the membranes were stained with Coomassie blue. Bead, glutathione–agarose beads alone; P, pellet; S, supernatant (10% of total). (D) Colocalization of EPSIN1 with clathrin. Protoplasts transformed with HA:EPSIN1 were fixed with paraglutaraldehyde, and the localization of HA:EPSIN1 and clathrin was examined by immunostaining with anti-HA and anti-clathrin antibodies, respectively. Bar = 20 μm.
Figure 5.
EPSIN1 Binds to VTI11. (A) Protein extracts were prepared from _VTI11:HA_- and _VTI12:HA_-transformed protoplasts and mixed with GST alone or GST:EPSIN1. EPSIN1-bound proteins were precipitated from the mixture with glutathione–agarose beads and analyzed by protein gel blotting using anti-HA antibody. (B) Coimmunoprecipitation of EPSIN1:GFP with VTI11:HA. Protein extracts from protoplasts cotransformed with VTI11:HA and EPSIN1:GFP were used for immunoprecipitation with anti-HA antibody. The immunoprecipitates were analyzed by protein gel blotting with anti-HA, anti-GFP, and anti-calreticulin antibodies. P, immunoprecipitate; S, supernatant; T, total protein extracts (5% of the input). (C) For binding experiments, protein extracts from protoplasts transformed with VTI11:HA were mixed with GST alone, GST:ENTH, and GST:EPSIN1ΔN. Proteins were precipitated with glutathione-agarose beads and analyzed by protein gel blotting using anti-HA antibody. The amount of the input proteins is indicated.
Figure 6.
EPSIN1 Binds to γ-ADR. (A) GFP_-fused γ_-ADR, α_-ADR_, and δ_-ADR_ were constructed and transiently expressed in protoplasts. Protein extracts were prepared from the transformed protoplasts and used for binding assays with GST:EPSIN1. The EPSIN1 binding proteins were precipitated from the reaction mixture and analyzed by protein gel blotting using anti-GFP antibody. P, pellet; S, supernatant (20% of total). (B) Pull-down of EPSIN1 proteins with MBP:γ-ADR(ear). MBP:γ-ADR(ear) was mixed with protein extracts from protoplasts transformed with the indicated constructs. MBP:γ-ADR(ear) binding proteins were precipitated with amylose resin, and the pellets were analyzed by protein gel blotting using anti-HA antibody. (C) The ear domains of γ-ADR and α-ADR were expressed as MBP fusion proteins in E. coli and used for binding experiments. Proteins were detected by protein gel blotting using anti-MBP antibody. (D) GST:EPSIN1 was mixed with MBP alone, MBP:γ-ADR(ear), or MBP:α-ADR(ear). The EPSIN1 binding proteins were precipitated from the reaction mixture and analyzed by protein gel blotting using anti-MBP antibody.
Figure 7.
EPSIN1 Binds to VSR1 via the ENTH Domain. (A) Protein extracts were prepared from protoplasts transformed with the indicated constructs and incubated with GST-fused EPSIN1. EPSIN1-bound proteins were precipitated with glutathione–agarose beads, and the pelleted proteins were analyzed by protein gel blotting using anti-HA antibody. P, pellet; S, supernatant (10% of total). (B) Protein extracts were prepared from protoplasts cotransformed with VSR1:HA and EPSIN1:GFP and used for immunoprecipitation with anti-HA antibody. The immunoprecipitates were analyzed by protein gel blotting using anti-HA, anti-GFP, and anti-PEP12 antibodies. P, immunoprecipitate; S, supernatant; T, total protein extracts (10% of input). (C) Protein extracts from protoplasts transformed with VSR1:HA were incubated with GST alone or GST-fused EPSIN1 proteins. EPSIN1 binding proteins were precipitated with glutathione–agarose beads, and the pelleted proteins were analyzed by protein gel blotting using anti-HA antibody.
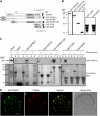
Figure 8.
EPSIN1 Is Necessary for the Efficient Vacuolar Trafficking of Spo:GFP. (A) Screen of an epsin1 mutant. T-DNA insertion in EPSIN1 was confirmed by PCR. HE, heterozygote; HO, homozygote; LB, left border primer; LP, left primer; M, size marker; RP, right primer. (B) Scheme of the epsin1 mutant. The position of the T-DNA insertion site is indicated. The numbers indicate nucleotide positions. Shaded boxes, exons; dark boxes, promoters or 3′ untranslated regions. (C) Total proteins were prepared from wild-type plants and heterozygotes and homozygotes of epsin1 plants and analyzed by protein gel blotting using anti-EPSIN1 antibody. The large subunit of the Rubisco complex (RbcL) stained with Coomassie blue was used as a loading control. (D) Trafficking assay of Spo:GFP in epsin1 protoplasts. Protoplasts from wild-type and epsin1 plants were transformed with Spo:GFP. Protein extracts prepared from protoplasts at various time points after transformation were analyzed by protein gel blotting using anti-GFP antibody. (E) Protein gel blot analysis of epsin1 mutants harboring HA:EPSIN1. Protein extracts were prepared from the T2 generation of epsin1 transgenic plants harboring HA:EPSIN1 and examined by protein gel blot analysis using anti-HA, anti-EPSIN1, and anti-BiP antibodies. C, epsin1 mutant; T1 to T3, independent transgenic plants harboring HA:EPSIN1 on the epsin1 background. (F) and (G) Protoplasts obtained from wild-type, epsin1, and epsin1 complemented with HA:EPSIN1 (EPSIN1/epsin1; T3) plants were transformed with Spo:GFP. (F) Protein extracts were obtained from protoplasts and analyzed by protein gel blotting using anti-GFP antibody. (G) Trafficking efficiency was determined as the ratio of the amount of the processed form to the total amount of Spo:GFP (processed plus precursor forms). Error bars indicate
sd
(n = 3). (H) Invertase:GFP secretion was not inhibited. Protoplasts obtained from epsin1 plants were transformed with invertase:GFP, and the secretion of invertase:GFP was examined at various time points after transformation. Protein extracts were prepared from the protoplasts, as well as from the media, and analyzed by protein gel blotting using anti-GFP antibody.
Similar articles
- EpsinR2 interacts with clathrin, adaptor protein-3, AtVTI12, and phosphatidylinositol-3-phosphate. Implications for EpsinR2 function in protein trafficking in plant cells.
Lee GJ, Kim H, Kang H, Jang M, Lee DW, Lee S, Hwang I. Lee GJ, et al. Plant Physiol. 2007 Apr;143(4):1561-75. doi: 10.1104/pp.106.095349. Epub 2007 Feb 2. Plant Physiol. 2007. PMID: 17277094 Free PMC article. - Actin filaments play a critical role in vacuolar trafficking at the Golgi complex in plant cells.
Kim H, Park M, Kim SJ, Hwang I. Kim H, et al. Plant Cell. 2005 Mar;17(3):888-902. doi: 10.1105/tpc.104.028829. Epub 2005 Feb 18. Plant Cell. 2005. PMID: 15722471 Free PMC article. - Trafficking of vacuolar proteins: the crucial role of Arabidopsis vacuolar protein sorting 29 in recycling vacuolar sorting receptor.
Kang H, Kim SY, Song K, Sohn EJ, Lee Y, Lee DW, Hara-Nishimura I, Hwang I. Kang H, et al. Plant Cell. 2012 Dec;24(12):5058-73. doi: 10.1105/tpc.112.103481. Epub 2012 Dec 21. Plant Cell. 2012. PMID: 23263768 Free PMC article. - [Regulatory mechanisms of the clathrin adaptor molecules AP-1 and GGAs].
Kametaka S, Bonifacino JS. Kametaka S, et al. Tanpakushitsu Kakusan Koso. 2008 Dec;53(16 Suppl):2046-52. Tanpakushitsu Kakusan Koso. 2008. PMID: 21038583 Review. Japanese. No abstract available. - Endosomal functions in plants.
Otegui MS, Spitzer C. Otegui MS, et al. Traffic. 2008 Sep;9(10):1589-98. doi: 10.1111/j.1600-0854.2008.00787.x. Epub 2008 Jul 9. Traffic. 2008. PMID: 18627577 Review.
Cited by
- EpsinR2 interacts with clathrin, adaptor protein-3, AtVTI12, and phosphatidylinositol-3-phosphate. Implications for EpsinR2 function in protein trafficking in plant cells.
Lee GJ, Kim H, Kang H, Jang M, Lee DW, Lee S, Hwang I. Lee GJ, et al. Plant Physiol. 2007 Apr;143(4):1561-75. doi: 10.1104/pp.106.095349. Epub 2007 Feb 2. Plant Physiol. 2007. PMID: 17277094 Free PMC article. - Homomeric interaction of AtVSR1 is essential for its function as a vacuolar sorting receptor.
Kim H, Kang H, Jang M, Chang JH, Miao Y, Jiang L, Hwang I. Kim H, et al. Plant Physiol. 2010 Sep;154(1):134-48. doi: 10.1104/pp.110.159814. Epub 2010 Jul 12. Plant Physiol. 2010. PMID: 20625000 Free PMC article. - Enrichment of hydroxylated C24- and C26-acyl-chain sphingolipids mediates PIN2 apical sorting at trans-Golgi network subdomains.
Wattelet-Boyer V, Brocard L, Jonsson K, Esnay N, Joubès J, Domergue F, Mongrand S, Raikhel N, Bhalerao RP, Moreau P, Boutté Y. Wattelet-Boyer V, et al. Nat Commun. 2016 Sep 29;7:12788. doi: 10.1038/ncomms12788. Nat Commun. 2016. PMID: 27681606 Free PMC article. - The cytosolic tail dipeptide Ile-Met of the pea receptor BP80 is required for recycling from the prevacuole and for endocytosis.
Saint-Jean B, Seveno-Carpentier E, Alcon C, Neuhaus JM, Paris N. Saint-Jean B, et al. Plant Cell. 2010 Aug;22(8):2825-37. doi: 10.1105/tpc.109.072215. Epub 2010 Aug 31. Plant Cell. 2010. PMID: 20807880 Free PMC article.
References
- Ahmed, S.U., Rojo, E., Kovaleva, V., Venkataraman, S., Dombrowski, J.E., Matsuoka, K., and Raikhel, N.V. (2000). The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 149 1335–1344. - PMC - PubMed
- Bassham, D.C., and Raikhel, N.V. (2000). Unique features of the plant vacuolar sorting machinery. Curr. Opin. Cell Biol. 12 491–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials