Estrogen-mediated post transcriptional down-regulation of P-glycoprotein in MDR1-transduced human breast cancer cells - PubMed (original) (raw)
Estrogen-mediated post transcriptional down-regulation of P-glycoprotein in MDR1-transduced human breast cancer cells
Kazuyoshi Mutoh et al. Cancer Sci. 2006 Nov.
Abstract
The human multidrug resistance gene 1 (MDR1) encodes the plasma membrane P-glycoprotein (P-gp/ABCB1) that functions as an efflux pump for various anticancer agents. We recently reported that estrogens down-regulate the expression of breast cancer resistance protein (BCRP/ABCG2). In our present study we demonstrate that estrogens also down-regulate P-gp expression in the MDR1-transduced, estrogen receptor alpha (ER-alpha)-positive human breast cancer cells, MCF-7/MDR and T-47D/MDR. The P-gp expression levels in MCF-7/MDR cells treated with 100 pM estradiol were found to be 10-20-fold lower than the levels in these same cells that were cultured without estradiol. In contrast, estradiol did not affect the P-gp expression levels in the ER-alpha-negative cancer cells, MDA-MB-231/MDR and NCI/ADR-RES. Estrone and diethylstilbestrol were also found to down-regulate P-gp in MCF-7/MDR cells, but progesterone treatment did not produce this effect. Tamoxifen reversed the estradiol-mediated down-regulation of P-gp in MCF-7/MDR cells, suggesting that ER-alpha activity is necessary for the effects of estradiol upon P-gp. However, estradiol was found not to alter the MDR1 transcript levels in either MCF-7/MDR and T-47D/MDR cells, suggesting that post-transcriptional mechanisms underlie its effects upon P-gp down-regulation. MCF-7/MDR cells also showed eight-fold higher sensitivity to vincristine when treated with 100 pM estradiol, than when treated with 1 pM estradiol. These results may serve to provide a better understanding of the expression control of ABC transporters, and possibly allow for the establishment of new cancer chemotherapy strategies that would control P-gp expression in breast cancer cells and thereby increase their sensitivity to MDR1-related anticancer agents.
Figures
Figure 1
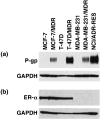
P‐glycoprotein (P‐gp) and estrogen receptor α (ER‐α) expression levels in cancer cell lines. For the detection of P‐gp, cell lysates (40 µg) were subjected to western blot analysis with the anti‐P‐gp monoclonal antibody, C219. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) levels were assessed as a loading control using the anti‐GAPDH monoclonal antibody. For the detection of ER‐α, whole cell lysates were subjected to western blot analysis with the anti‐ER‐α monoclonal antibody, NCL‐ER‐‐6F11, and anti‐GAPDH monoclonal antibody.
Figure 2
The effects of E2 on P‐glycoprotein (P‐gp) expression. The indicated cells were cultured in basal medium in the absence or presence of the indicated concentrations of E2 for 4 days. Cell lysates (40 µg) were then subjected to western blot analysis with the anti‐P‐gp monoclonal antibody, C219. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) levels were assessed as a loading control using the anti‐GAPDH monoclonal antibody.
Figure 3
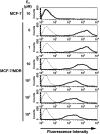
Analysis of the cell surface expression of P‐glycoprotein (P‐gp) using fluorescence‐activated cell sorting (FACS) analysis. MCF‐7/MDR cells were cultured in basal medium in the absence or presence of the indicated concentrations of E2 for 4 days. MCF‐7/MDR cells were then harvested, incubated with or without the biotinylated F(ab′)2 fragment of the anti‐P‐gp monoclonal antibody, MRK16, and then incubated with R‐phycoerythrin‐conjugated streptavidin. After washing the cells, the fluorescence intensities were determined using FACSCalibur. The bold and dotted lines indicate that the cells were incubated with and without MRK16, respectively.
Figure 4
The effects of estrogens, progesterone and tamoxifen on P‐glycoprotein (P‐gp) expression levels. MCF‐7/MDR cells were cultured in basal medium in the absence or presence of the indicated concentrations of each agent for 4 days. Cell lysates (40 µg) were then subjected to western blot analysis with the anti‐P‐gp monoclonal antibody, C219, and anti‐glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) monoclonal antibody to normalize for protein loading control. (a) Effects of estrogens on P‐gp expression. (b) Effects of progesterone on P‐gp expression. (c) Effects of tamoxifen on P‐gp expression in the absence of E2 (left panel) and the reversal effects of tamoxifen on the E2‐mediated down‐regulation of P‐gp (right panel).
Figure 5
Reverse transcription polymerase chain reaction (RT‐PCR) analysis of the MDR1 mRNA expression levels in both MCF‐7/MDR and T‐47D/MDR cells. Cells were cultured in basal medium in the absence or presence of the indicated concentrations of E2 for 4 days. Exponentially growing cells were harvested and total RNA was extracted. First‐strand cDNAs were synthesized with 0.3 µg of total RNA, and an MDR1 cDNA fragment (702 bp) was subsequently amplified by PCR using the indicated cycles. The amplification of GAPDH mRNA (551 bp fragment) was performed as an internal control.
Figure 6
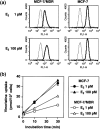
Analysis of rhodamine 123 and [3H]vincristine uptake in E2‐treated MCF‐7/MDR cells. (a) Rhodamine 123 uptake. MCF‐7 and MCF‐7/MDR cells were cultured in basal medium in the presence of either 1 or 100 pM E2 for 4 days. After trypsinization, the cells were incubated with (––) or without (‐‐‐) 100 nM rhodamine 123 in basal medium supplemented with the same concentrations of E2 for 20 min. After washing of the cells, the cellular uptake of rhodamine 123 was measured by FACSCalibur. (b) [3H]Vincristine uptake. MCF‐7 (▴•) and MCF‐7/MDR (▵○) cells were cultured in basal medium in the presence of either 1 pM (•○) or 100 pM (▴▵) E2 for 4 days. The cells were then washed and incubated with 50 nM [3H]vincristine for 0.5, 10, and 30 min in basal medium supplemented with the same concentration of E2. After washing of the cells, the cells were lyzed, and [3H]vincristine uptake was measured by liquid scintillation. Data are the mean ± SD values from triplicate determinations. Where a horizontal bar is not shown, the SD is low and contained within the symbol.
Figure 7
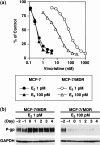
Drug sensitivity levels of E2‐treated MCF‐7/MDR cells. (a) Drug sensitivity to vincristine. MCF‐7 cells (•▴) and MCF‐7/MDR cells (○▵) were cultured in basal medium in the presence of either 1 pM (•○) or 100 pM (▴▵) E2 for 2 days. Vincristine was then added to the cultures and the cells were incubated for an additional 4 days. Cell numbers were determined using a cell counter. Data are the mean ± SD values from triplicate determinations. Where a horizontal bar is not shown, the SD is low and contained within the symbol. (b) Timecourse analysis of the P‐gp expression levels in E2‐treated MCF‐7/MDR cells. MCF‐7/MDR cells were cultured in basal medium in the presence of either 1 or 100 pM E2 for up to 6 days. Cell pellets were obtained each day and cell lysates were subjected to western blot analysis with the anti‐P‐glycoprotein (P‐gp) monoclonal antibody, C219.
Similar articles
- Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2.
Imai Y, Ishikawa E, Asada S, Sugimoto Y. Imai Y, et al. Cancer Res. 2005 Jan 15;65(2):596-604. Cancer Res. 2005. PMID: 15695404 - Survivin transcription is associated with P-glycoprotein/MDR1 overexpression in the multidrug resistance of MCF-7 breast cancer cells.
Liu F, Liu S, He S, Xie Z, Zu X, Jiang Y. Liu F, et al. Oncol Rep. 2010 May;23(5):1469-75. doi: 10.3892/or_00000786. Oncol Rep. 2010. PMID: 20372866 - Reversal of P-glycoprotein-mediated multidrug resistance is induced by saikosaponin D in breast cancer MCF-7/adriamycin cells.
Li C, Guan X, Xue H, Wang P, Wang M, Gai X. Li C, et al. Pathol Res Pract. 2017 Jul;213(7):848-853. doi: 10.1016/j.prp.2017.01.022. Epub 2017 Feb 3. Pathol Res Pract. 2017. PMID: 28554760 - Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies.
Amiri-Kordestani L, Basseville A, Kurdziel K, Fojo AT, Bates SE. Amiri-Kordestani L, et al. Drug Resist Updat. 2012 Feb-Apr;15(1-2):50-61. doi: 10.1016/j.drup.2012.02.002. Epub 2012 Mar 29. Drug Resist Updat. 2012. PMID: 22464282 Free PMC article. Review. - Intracellular trafficking of P-glycoprotein.
Fu D, Arias IM. Fu D, et al. Int J Biochem Cell Biol. 2012 Mar;44(3):461-4. doi: 10.1016/j.biocel.2011.12.009. Epub 2011 Dec 24. Int J Biochem Cell Biol. 2012. PMID: 22212176 Free PMC article. Review.
Cited by
- Asclepiasterol, a novel C21 steroidal glycoside derived from Asclepias curassavica, reverses tumor multidrug resistance by down-regulating P-glycoprotein expression.
Yuan WQ, Zhang RR, Wang J, Ma Y, Li WX, Jiang RW, Cai SH. Yuan WQ, et al. Oncotarget. 2016 May 24;7(21):31466-83. doi: 10.18632/oncotarget.8965. Oncotarget. 2016. PMID: 27129170 Free PMC article. - Effect of the novel antipsychotic drug perospirone on P-glycoprotein function and expression in Caco-2 cells.
Zhou YG, Li KY, Li HD. Zhou YG, et al. Eur J Clin Pharmacol. 2008 Jul;64(7):697-703. doi: 10.1007/s00228-008-0487-5. Epub 2008 May 14. Eur J Clin Pharmacol. 2008. PMID: 18478216 - Association between ABCB1, ABCG2 carrier protein and COX-2 enzyme gene polymorphisms and breast cancer risk in a Turkish population.
Zeliha KP, Dilek O, Ezgi O, Halil K, Cihan U, Gul O. Zeliha KP, et al. Saudi Pharm J. 2020 Feb;28(2):215-219. doi: 10.1016/j.jsps.2019.11.024. Epub 2019 Dec 7. Saudi Pharm J. 2020. PMID: 32042261 Free PMC article. - Oestrogen enhances cardiotoxicity induced by Sunitinib by regulation of drug transport and metabolism.
Harvey PA, Leinwand LA. Harvey PA, et al. Cardiovasc Res. 2015 Jul 1;107(1):66-77. doi: 10.1093/cvr/cvv152. Epub 2015 May 25. Cardiovasc Res. 2015. PMID: 26009590 Free PMC article. - Graphene nanoribbons elicit cell specific uptake and delivery via activation of epidermal growth factor receptor enhanced by human papillomavirus E5 protein.
Mullick Chowdhury S, Manepalli P, Sitharaman B. Mullick Chowdhury S, et al. Acta Biomater. 2014 Oct;10(10):4494-504. doi: 10.1016/j.actbio.2014.06.030. Epub 2014 Jun 27. Acta Biomater. 2014. PMID: 24980059 Free PMC article.
References
- Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P‐glycoprotein genes in multidrug‐resistant mammalian cell lines. Nature 1985; 316: 817–19. - PubMed
- Chen CJ, Chin JE, Ueda K et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (P‐glycoprotein) gene from multidrug‐resistant human cells. Cell 1986; 47: 381–9. - PubMed
- Shen DW, Fojo A, Chin JE et al. Human multidrug‐resistant cell lines: increased MDR1 expression can precede gene amplification. Science 1986; 232: 643–5. - PubMed
- Gottesman MM, Hrycyna CA, Schoenlein PV, Germamm UA, Pastan I. Genetic analysis of the multidrug transporter. Annu Rev Genet 1995; 29: 607–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous