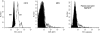Intravascular neutrophil activation due to carbon monoxide poisoning - PubMed (original) (raw)
Intravascular neutrophil activation due to carbon monoxide poisoning
Stephen R Thom et al. Am J Respir Crit Care Med. 2006.
Abstract
Rationale: We hypothesized that platelet-neutrophil interactions occur as a result of acute carbon monoxide (CO) poisoning, and subsequent neutrophil activation triggers events that cause neurologic sequelae.
Objectives: To identify platelet-neutrophil interactions and neutrophil activation in patients and in animal models, and to establish the association between these intravascular events and changes linked to CO-mediated neurologic sequelae in an animal model.
Measurements and main results: Blood was obtained from 50 consecutive patients. Abnormalities were variable depending on the carboxyhemoglobin level at study admission and duration of CO exposure. Platelet-neutrophil aggregates were detected and plasma myeloperoxidase (MPO) concentration was significantly elevated in those with confirmed CO poisoning. Among patients exposed to CO for over 3 h, flow cytometry scans of neutrophils revealed increased surface expression of CD18 and, in some groups, MPO on the cell surface. Animal models revealed consistent evidence of platelet-neutrophil aggregates, neutrophil activation and surface MPO, and plasma MPO elevation. MPO was deposited along the brain vascular lining and colocalized with nitrotyrosine. CO poisoning caused abnormalities in the charge pattern of myelin basic protein (MBP), changes linked to adaptive immunologic responses responsible for neurologic sequelae in this model. Changes did not occur in thrombocytopenic rats, those receiving tirofiban to inhibit platelet-neutrophil interactions, or those receiving L-nitroarginine methyl ester to inhibit nitric oxide synthesis. Alterations in MBP did not occur in CO-poisoned knockout mice lacking MPO.
Conclusions: Acute CO poisoning causes intravascular neutrophil activation due to interactions with platelets. MPO liberated by neutrophils mediates perivascular oxidative stress, which is linked to immune-mediated neurologic sequelae.
Figures
**Figure 1.
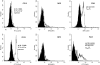
Representative neutrophil histograms for human control subjects and CO-poisoned patients. The top three panels show results for cells from a control person (black curves) and results for cells obtained from a 26-yr-old woman who presented to the emergency department after exposure for approximately 0.6 h to smoke in a house fire. Her presenting COHb level was 36%, and she suffered no loss of consciousness. A small shift in surface expression of CD18 and MPO was observed, and there was a moderate presence of platelets attached to neutrophils based on neutrophil surface expression of CD61, the β3 component of the platelet glycoprotein αIIbβ3 integrin. The lower three panels show results for a different control person from the one in the upper panels, and surface expression for neutrophils from a 46-yr-old man exposed to CO from a faulty home heater for approximately 6.5 h. His COHb level on admission was 35% and he had suffered transient loss of consciousness. His neutrophils exhibited a substantial increase in surface CD18 expression, mild presence of myeloperoxidase (MPO), and activated platelet glycoprotein αIIbβ3 integrin. FITC = fluorescein isothiocyanate; RPE = R-phycoerythrin.
**Figure 2.
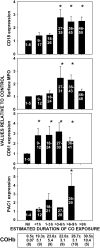
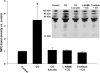
Expression of selected proteins on neutrophils from suspected CO-exposed patients relative to control cells run on the same day. The “nil” group indicates results using neutrophils from patients in whom CO poisoning was not confirmed. Other results are categorized according to the estimated duration of CO poisoning that a patient suffered. Surface CD18 was assessed using cells from all 50 consecutive patients. MPO was measured on the surface of neutrophils from 32 patients, CD61 on cells from 25 patients, and PAC1 on cells from 17 patients. Data are mean ± SE; *p < 0.05, analysis of variance (ANOVA). The numbers within each bar refer to individual patients described in Table E1. The mean COHb (± SE) for each group (n = total number of patients in each group) is shown at the bottom of the figure.
**Figure 3.
Representative histograms for expression of markers on the surface of rat neutrophils. Panels show results for cells from a control rat (black curves) and results for cells obtained from a rat poisoned with CO and killed 90 min later. After CO poisoning, the cells exhibited increases in surface expression of CD18 and MPO, and a mild elevation in platelets adherent to the cell surface.
**Figure 4.
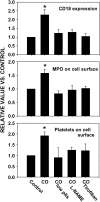
Relative surface CD18 expression, surface MPO presence, and platelet–neutrophil aggregates for rat neutrophils. Data were obtained for cells from control rats, CO-poisoned untreated rats, those first injected with antiserum to render them thrombocytopenic, rats injected with
l
-nitroarginine methylester (
l
-NAME), or rats injected with tirofiban prior to CO poisoning (n = 4 rats in each group). Data are mean ± SE; *p < 0.05, ANOVA. plts = platelets.
**Figure 5.
Plasma MPO content in rats. Data were obtained from control rats, untreated CO-poisoned rats, those first injected with antiserum to render them thrombocytopenic, or rats injected with
l
-NAME or tirofiban prior to CO poisoning. The figure shows a typical Western blot and quantitative evaluations of the 60-kD band density using 50 μg of plasma protein from rats (n = 4 rats in each group). Data are mean ± SE; *p < 0.05, ANOVA.
**Figure 6.
Content of MPO and CD66 protein in brain homogenates from control and CO-poisoned rats. Data were obtained from control rats, untreated CO-poisoned rats, those first injected with antiserum to render them thrombocytopenic, or rats injected with
l
-NAME or tirofiban prior to CO poisoning. Western blots were prepared and probed with antibody to MPO and to the structural neutrophil CD66 protein (n = 4 rats in each group). Data are the ratio of band density (MPO/CD66), mean ± SE; *p < 0.05, ANOVA.
**Figure 7.
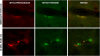
Immunohistochemical images of brain sections from rats killed immediately or at 90 min after CO poisoning. Dual staining was performed with an anti-MPO antibody counterstained with Cy3 and an anti–nitrotyrosine antibody counterstained with FITC. Image processing allowed both stains to be overlaid and yellow demonstrated exact colocalization of staining.
**Figure 8.
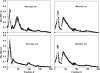
Column chromatography analysis. Panels show the elution patterns of myelin basic protein from cation exchange chromatography columns using acid-soluble brain material obtained from control rats, rats killed 90 min after CO poisoning, and CO-poisoned rats first injected with antiserum to render them thrombocytopenic or neutropenic, or rats injected with
l
-NAME or tirofiban. Higher fraction numbers indicate more positively charged proteins. Data points are mean ± SE; n = 4 rats in each group.
**Figure 9.
Column chromatography analysis of acid-soluble material from mouse brain. Panels show the protein elution patterns for wild-type and MPO-knockout (MPO-KO) mice breathing air (control) and at 90 min after CO poisoning. Data points are mean ± SE; n = 3 mice in each group.
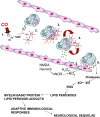
**Figure 10.
Schematic illustrating the proposed mechanism for immune-mediated neurologic sequelae. Events numbered 1 through 6 are described in the current study. Additional elements are based on results from published studies (20, 34–36, 38, 41, 51). 1: CO binds to platelet hemoproteins and the competition with intraplatelet .NO increases .NO efflux (first reported in Reference 20). 2: Platelet-derived .NO reacts with neutrophil-derived superoxide (O2−.), giving rise to reactive species that activate platelets and cause platelet–neutrophil aggregates (Step 3). 4: Ongoing interactions involving reactive products and adhesion molecules cause firm aggregation and stimulate intravascular neutrophil degranulation. 5: MPO is deposited along the vascular lining and some is transcytosed to the subendothelial matrix, where it reacts with nitrite generated by N-methyl D-aspartate (NMDA) neurons. Products from MPO-mediated reactions cause endothelial cell activation, facilitating firm neutrophil adhesion and further degranulation. 6: Neutrophil-derived proteases react with endothelial cell xanthine dehydrogenase (XD), converting it to xanthine oxidase (XO). Reactive oxygen species (ROS) initiate lipid peroxidation and adducts interact with brain myelin basic protein. The altered myelin basic protein triggers an adaptive immunologic response that causes neurologic dysfunction. nNOS = neuronal nitric oxide synthase.
Similar articles
- Neuronal nitric oxide synthase and N-methyl-D-aspartate neurons in experimental carbon monoxide poisoning.
Thom SR, Fisher D, Zhang J, Bhopale VM, Cameron B, Buerk DG. Thom SR, et al. Toxicol Appl Pharmacol. 2004 Feb 1;194(3):280-95. doi: 10.1016/j.taap.2003.09.017. Toxicol Appl Pharmacol. 2004. PMID: 14761684 - Nitric oxide released by platelets inhibits neutrophil B2 integrin function following acute carbon monoxide poisoning.
Thom SR, Ohnishi ST, Ischiropoulos H. Thom SR, et al. Toxicol Appl Pharmacol. 1994 Sep;128(1):105-10. doi: 10.1006/taap.1994.1186. Toxicol Appl Pharmacol. 1994. PMID: 7521544 - Nitric oxide production and perivascular nitration in brain after carbon monoxide poisoning in the rat.
Ischiropoulos H, Beers MF, Ohnishi ST, Fisher D, Garner SE, Thom SR. Ischiropoulos H, et al. J Clin Invest. 1996 May 15;97(10):2260-7. doi: 10.1172/JCI118667. J Clin Invest. 1996. PMID: 8636405 Free PMC article. - Carbon monoxide intoxication.
Bleecker ML. Bleecker ML. Handb Clin Neurol. 2015;131:191-203. doi: 10.1016/B978-0-444-62627-1.00024-X. Handb Clin Neurol. 2015. PMID: 26563790 Review. - Carbon monoxide poisoning: systemic manifestations and complications.
Choi IS. Choi IS. J Korean Med Sci. 2001 Jun;16(3):253-61. doi: 10.3346/jkms.2001.16.3.253. J Korean Med Sci. 2001. PMID: 11410684 Free PMC article. Review.
Cited by
- Combined application of dexamethasone and hyperbaric oxygen therapy yields better efficacy for patients with delayed encephalopathy after acute carbon monoxide poisoning.
Xiang W, Xue H, Wang B, Li Y, Zhang J, Jiang C, Liang F, Pang J, Yu L. Xiang W, et al. Drug Des Devel Ther. 2017 Feb 23;11:513-519. doi: 10.2147/DDDT.S126569. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28260864 Free PMC article. Clinical Trial. - Clinical and Echocardiographic Predictors for the Presence of Late Gadolinium Enhancement on Cardiac Magnetic Resonance Imaging in Patients with Carbon Monoxide Poisoning.
Cho DH, Son JW, Kim YI, Lim J, Jeon HS, Ko SM, Cha YS. Cho DH, et al. Diagnostics (Basel). 2023 Dec 27;14(1):60. doi: 10.3390/diagnostics14010060. Diagnostics (Basel). 2023. PMID: 38201369 Free PMC article. - Carbon Monoxide Poisoning: From Microbes to Therapeutics.
Dent MR, Rose JJ, Tejero J, Gladwin MT. Dent MR, et al. Annu Rev Med. 2024 Jan 29;75:337-351. doi: 10.1146/annurev-med-052422-020045. Epub 2023 Aug 15. Annu Rev Med. 2024. PMID: 37582490 Free PMC article. Review. - Carbon Monoxide as a Potential Therapeutic Agent: A Molecular Analysis of Its Safety Profiles.
Bansal S, Liu D, Mao Q, Bauer N, Wang B. Bansal S, et al. J Med Chem. 2024 Jun 27;67(12):9789-9815. doi: 10.1021/acs.jmedchem.4c00823. Epub 2024 Jun 12. J Med Chem. 2024. PMID: 38864348 Free PMC article. Review.
References
- Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR. Carbon monoxide: a public health perspective. Toxicology 2000;145:1–14. - PubMed
- Coburn RF, Forman HJ. Carbon monoxide toxicity. In: Fishman AP, Farki LE, Geiger SR, editors. Handbook of physiology. Baltimore: Williams & Wilkins; 1987. pp. 439–456.
- Piantadosi CA, Tatro L, Zhang J. Hydroxyl radical production in the brain after CO hypoxia in rats. Free Radic Biol Med 1995;18:603–609. - PubMed
- Okeda R, Funata N, Song SJ, Higashino F, Takano T, Yokoyama K. Comparative study pathogenesis of selective cerebral lesions in carbon monoxide poisoning and nitrogen hypoxia in cats. Acta Neuropathol (Berl) 1982;56:265–272. - PubMed
- Piantadosi CA, Zhang J, Levin ED, Folz RJ, Schmechel DE. Apoptosis and delayed neuronal damage after carbon monoxide poisoning in the rat. Exp Neurol 1997;147:103–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous