Recapitulate development to promote axonal regeneration: good or bad approach? - PubMed (original) (raw)
Review
Recapitulate development to promote axonal regeneration: good or bad approach?
Marie T Filbin. Philos Trans R Soc Lond B Biol Sci. 2006.
Abstract
In the past decade there has been an explosion in our understanding, at the molecular level, of why axons in the adult, mammalian central nervous system (CNS) do not spontaneously regenerate while their younger counterparts do. Now a number of inhibitors of axonal regeneration have been described, some of the receptors they interact with to transduce the inhibitory signal are known, as are some of the steps in the signal transduction pathway that is responsible for inhibition. In addition, developmental changes in the environment and in the neurons themselves are also now better understood. This knowledge in turn reveals novel, putative sites for drug development and therapeutic intervention after injury to the brain and spinal cord. The challenge now is to determine which of these putative treatments are the most effective and if they would be better applied in combination rather than alone. In this review I will summarize what we have learnt about these molecules and how they signal. Importantly, I will also describe approaches that have been shown to block inhibitors and encourage regeneration in vivo. I will also speculate on what the differences are between the neonatal and adult CNS that allow the former to regenerate and the latter not to.
Figures
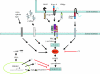
Figure 1
Inhibitors of axonal regeneration, their receptors and how they signal. Three myelin inhibitors, MAG, Nogo-66 and OMgp, all interact with the NgR/Lingo/p75 (TROY) receptor complex. The receptors for Amino-Nogo and CSPGs are not known. All the inhibitors exert inhibition by activating Rho. Inactivation of Rho or elevation of cAMP each overcome all the inhibitors simultaneously. The cAMP effect is CREB- and transcription-dependent. ArgI is up-regulated, resulting in an increase in polyamine synthesis. Polyamines can block inhibition.
Similar articles
- Concept and molecular basis of axonal regeneration after central nervous system injury.
Muramatsu R, Yamashita T. Muramatsu R, et al. Neurosci Res. 2014 Jan;78:45-9. doi: 10.1016/j.neures.2013.07.002. Epub 2013 Jul 26. Neurosci Res. 2014. PMID: 23896200 Review. - Enhancing intrinsic growth capacity promotes adult CNS regeneration.
Yang P, Yang Z. Yang P, et al. J Neurol Sci. 2012 Jan 15;312(1-2):1-6. doi: 10.1016/j.jns.2011.08.037. Epub 2011 Sep 16. J Neurol Sci. 2012. PMID: 21924742 Review. - Spinal cord repair: strategies to promote axon regeneration.
McKerracher L. McKerracher L. Neurobiol Dis. 2001 Feb;8(1):11-8. doi: 10.1006/nbdi.2000.0359. Neurobiol Dis. 2001. PMID: 11162236 Review. - Can regeneration be promoted within the spinal cord?
Kuffler D. Kuffler D. P R Health Sci J. 2000 Sep;19(3):241-52. P R Health Sci J. 2000. PMID: 11076370 Review. - L1, beta1 integrin, and cadherins mediate axonal regeneration in the embryonic spinal cord.
Blackmore M, Letourneau PC. Blackmore M, et al. J Neurobiol. 2006 Dec;66(14):1564-83. doi: 10.1002/neu.20311. J Neurobiol. 2006. PMID: 17058193
Cited by
- Regulation of Adult CNS Axonal Regeneration by the Post-transcriptional Regulator Cpeb1.
Lou WP, Mateos A, Koch M, Klussman S, Yang C, Lu N, Kumar S, Limpert S, Göpferich M, Zschaetzsch M, Sliwinski C, Kenzelmann M, Seedorf M, Maillo C, Senis E, Grimm D, Puttagunta R, Mendez R, Liu K, Hassan BA, Martin-Villalba A. Lou WP, et al. Front Mol Neurosci. 2018 Jan 12;10:445. doi: 10.3389/fnmol.2017.00445. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29379413 Free PMC article. - The DLK signalling pathway--a double-edged sword in neural development and regeneration.
Tedeschi A, Bradke F. Tedeschi A, et al. EMBO Rep. 2013 Jul;14(7):605-14. doi: 10.1038/embor.2013.64. Epub 2013 May 17. EMBO Rep. 2013. PMID: 23681442 Free PMC article. Review. - Long-distance growth and connectivity of neural stem cells after severe spinal cord injury.
Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Lu P, et al. Cell. 2012 Sep 14;150(6):1264-73. doi: 10.1016/j.cell.2012.08.020. Cell. 2012. PMID: 22980985 Free PMC article. - Three-Dimensional Environment Sustains Morphological Heterogeneity and Promotes Phenotypic Progression During Astrocyte Development.
Balasubramanian S, Packard JA, Leach JB, Powell EM. Balasubramanian S, et al. Tissue Eng Part A. 2016 Jun;22(11-12):885-98. doi: 10.1089/ten.TEA.2016.0103. Tissue Eng Part A. 2016. PMID: 27193766 Free PMC article. - Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage.
Tan HB, Zhong YS, Cheng Y, Shen X. Tan HB, et al. Int J Ophthalmol. 2011;4(6):652-7. doi: 10.3980/j.issn.2222-3959.2011.06.16. Epub 2011 Dec 18. Int J Ophthalmol. 2011. PMID: 22553739 Free PMC article.
References
- Bregman B.S, Goldberger M.E. Anatomical plasticity and sparing of function after spinal cord damage in neonatal cats. Science. 1982;217:553–555. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous