Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability - PubMed (original) (raw)
Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability
Cyrille J Cohen et al. Cancer Res. 2006.
Abstract
Little is known about the biology of murine T-cell receptors (TCR) expressed in human cells. We recently observed that a murine anti-human p53 TCR is highly functional when expressed in human lymphocytes. Herein, we compare human and mouse TCR function and expression to delineate the molecular basis for the apparent superior biological activity of murine receptors in human T lymphocytes. To this end, we created hybrid TCRs where we swapped the original constant regions with either human or mouse ones, respectively. We showed that murine or "murinized" receptors were overexpressed on the surface of human lymphocytes compared with their human/humanized counterparts and were able to mediate higher levels of cytokine secretion when cocultured with peptide-pulsed antigen-presenting cells. Preferential pairing of murine constant regions and improved CD3 stability seemed to be responsible for these observations. These enhanced biological properties translated into significantly greater antitumor response mediated by TCR with mouse constant regions. Furthermore, we were able to circumvent the natural low avidity of class I MHC TCR in CD4(+) cells by introducing the murinized TCR into CD4(+) lymphocytes, giving them the ability to recognize melanoma tumors. These findings have implications for human TCR gene transfer therapy and may provide new insights into the biology of the TCR/CD3 complex.
Figures
Figure 1
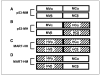
Schematic representation of the TCR chains and their hybrids. A, murine anti-p53 TCR, p53-MM. B, its humanized version, p53-MH, with mouse variable regions linked to human constant regions. Human anti-MART-1 TCR, MART-HH (C), and its murinized hybrid, MART-HM (D), with human variable regions linked to murine constant regions.
Figure 2
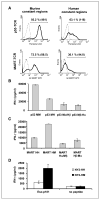
A, comparison of the surface expression of the original TCRs and their hybrids. OKT-3-stimulated PBLs were electroporated with mRNA encoding the different TCR chains and assessed by FACS. Twenty-four hours after electroporation, we assessed p53 pentamer binding for p53-MM or p53-MH and MART-1 tetramer binding for MART-HM or MART-HH. Percentage of positive cells as well as the relative MFI (inside parentheses). B to D, recognition of peptide pulsed cells by original and hybrid TCRs. B, human PBLs were electroporated with the p53-MM, the p53-MH, the α chain of the p53-MM-Mα + the β chain of the p53-MH-Hβ (Mα/Hβ), or the opposite noted as Mβ/Hα. The electroporated cells were then cocultured for 16 hours with T2 cells that were pulsed at 1 μmol/L with specific peptide (p53264-272) or nonspecific peptides (control; data not shown). The concentration of IFN-γ secreted in the medium was measured using an ELISA procedure. C, PBLs were electroporated with the MART-HH, MART-HM, MART-Hα/Mβ, and MART-Hβ/Mα and then cocultured with cultured for 16 hours with T2 cells that were pulsed at 1 μmol/L with specific peptide (MART-1/27L26-35) or nonspecific peptides (control; data not shown). IFN-γ secretion in cultures with T2 pulsed with control peptides (gp100-209, + gp100-280, p53149-157, and HBVc) was ≤200 pg/mL (data not shown). D, CD4 enriched cells were electroporated with mRNA encoding the α and β chains of NY2-HH, a HLA-DP4-restricted NY-ESO-1 TCR, or its mouse hybrid, NY2-HM. NY-ESO-1161-180 peptide-pulsed HLA-DP4+ EBV-B line (DK-EBV-B) as well as nonpulsed cells as a control were cocultured overnight with TCR RNA electroporated CD4 T cells. IFN-γ secretion was detected by ELISA.
Figure 3
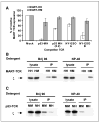
A, TCR competition assay. TCR-deficient Jurkat RT3-T3.5 cells were electroporated with 1 μg of each chain of the MART-HH (white columns) or MART-HM (gray columns) along with 1 μg of each chain of the competitor TCR [for p53-MH, we also used an additional amount (i.e., 0.2 μg) indicated as _p53-MH (0.2)_]. Twenty-four hours after electroporation, the cells were stained with MART-1 tetramer and the percentage of MART-1 TCR relative expression was calculated by dividing percentage of tetramer-positive cells of a given sample by that of the control sample (without competitor TCR). All the differences were statistically significant based on Student’s t test (P < 0.005). B, enhanced CD3/TCR stability mediated by MART-HM. TCR-deficient Jurkat RT3-T3.5 cells were electroporated with the MART-HH or MART-HM. Twenty-four hours after electroporation, cells were lysed in two different detergents (Brij96, mild detergent; NP40, strong detergent). The TCR was immunoprecipitated with a Vβ12 and the precipitate was subjected to a Western blot analysis for CD3ζ. As a control for protein loading (CD3ζ), we used the unprecipitated cell lysate. Representative of one of three independent experiments. C, enhanced CD3/TCR stability mediated by p53-MM. Similarly, we electroporated Jurkat RT3-T3.5 cells with either p53-MM or p53-MH and immunoprecipitated the TCR with an anti-murine Vβ3 antibody under different detergent conditions. We then subjected the precipitated to a Western blot analysis for CD3ζ and used the unprecipitated lysate as control.
Figure 4
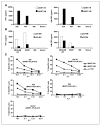
Enhanced recognition of tumor lines. A, human PBLs expressing either the MART-HH (white columns) or the MART-HM (black columns) were cocultured with the HLA-A2+ 526 and 624, the HLA-A2− 888 melanoma cell lines, and the HLA-A2+ Saos-2 osteosarcoma line. Twenty-four hours after the beginning of the coculture, the concentration of IFN-γ and GM-CSF secreted in the medium was measured using an ELISA procedure. Representative of 1 of 10 independent experiments each done with different donors. B, similarly, human PBLs expressing either the p53-MM (white columns) or the p53-MH (black columns) were cocultured with the p53+/HLA-A2+ MDA-MB-231, H2087 tumors, and the p53−/HLA-A2+ Saos-2 and HLA-A2− 888 control cell lines. Twenty-four hours after the beginning of the coculture, the concentration of IFN-γ and GM-CSF secreted in the medium was determined by ELISA. C, specific killing of tumor cell lines. CD8+ purified human PBLs expressing the MART-HH (triangles), the MART-HM (squares), or mock electroporated (stars) were cocultured for 3 hours with the indicated tumor cell lines labeled previously with 51Cr. Specific lysis was measured at the E:T ratios (specific release − spontaneous release) / (total release − spontaneous release). We used the following HLA-A2+/melanoma cell lines: 526, 624.38, and 624 as well as the HLA-A2−/melanoma 938 or HLA-A2+/osteosarcoma Saos-2 as control lines.
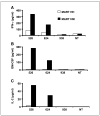
Figure 5
Increased antigen specificity in CD4+ cells expressing MART-HM. Purified CD4+ human PBLs expressing either the MART-HH (white columns) or the MART-HM (black columns) were cocultured with the HLA-A2+/melanoma cell lines 526 and 624, the HLA-A2−/melanoma 938, or without target (NT). The concentration of IFN-γ (A), GM-CSF (B), and IL-2 (C) secreted in the medium was measured using an ELISA procedure.
Similar articles
- Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells.
Sommermeyer D, Uckert W. Sommermeyer D, et al. J Immunol. 2010 Jun 1;184(11):6223-31. doi: 10.4049/jimmunol.0902055. Epub 2010 May 5. J Immunol. 2010. PMID: 20483785 - Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond.
Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Cohen CJ, et al. Cancer Res. 2007 Apr 15;67(8):3898-903. doi: 10.1158/0008-5472.CAN-06-3986. Cancer Res. 2007. PMID: 17440104 Free PMC article. - CD3 zeta dependence of the CD2 pathway of activation in T lymphocytes and natural killer cells.
Moingeon P, Lucich JL, McConkey DJ, Letourneur F, Malissen B, Kochan J, Chang HC, Rodewald HR, Reinherz EL. Moingeon P, et al. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1492-6. doi: 10.1073/pnas.89.4.1492. Proc Natl Acad Sci U S A. 1992. PMID: 1346934 Free PMC article. - Interplay between the TCR/CD3 complex and CD4 or CD8 in the activation of cytotoxic T lymphocytes.
de Vries JE, Yssel H, Spits H. de Vries JE, et al. Immunol Rev. 1989 Jun;109:119-41. doi: 10.1111/j.1600-065x.1989.tb00022.x. Immunol Rev. 1989. PMID: 2527803 Review. - From pathology to physiology of the human T-lymphocyte receptor.
Regueiro JR, Timón M, Pérez-Aciego P, Corell A, Martín-Vílla JM, Rodríguez-Gallego C, Góngora R, Arnaiz-Villena A. Regueiro JR, et al. Scand J Immunol. 1992 Sep;36(3):363-9. doi: 10.1111/j.1365-3083.1992.tb02950.x. Scand J Immunol. 1992. PMID: 1387725 Review.
Cited by
- Rapid parallel reconstruction and specificity screening of hundreds of T cell receptors.
Afeyan AB, Wu CJ, Oliveira G. Afeyan AB, et al. Nat Protoc. 2024 Nov 8. doi: 10.1038/s41596-024-01061-4. Online ahead of print. Nat Protoc. 2024. PMID: 39516267 Review. - Focusing on CD8+ T-cell phenotypes: improving solid tumor therapy.
Yao Z, Zeng Y, Liu C, Jin H, Wang H, Zhang Y, Ding C, Chen G, Wu D. Yao Z, et al. J Exp Clin Cancer Res. 2024 Sep 28;43(1):266. doi: 10.1186/s13046-024-03195-5. J Exp Clin Cancer Res. 2024. PMID: 39342365 Free PMC article. Review. - Engineered allogeneic T cells decoupling T-cell-receptor and CD3 signalling enhance the antitumour activity of bispecific antibodies.
Kapetanovic E, Weber CR, Bruand M, Pöschl D, Kucharczyk J, Hirth E, Dietsche C, Khan R, Wagner B, Belli O, Vazquez-Lombardi R, Castellanos-Rueda R, Di Roberto RB, Kalinka K, Raess L, Ly K, Rai S, Dittrich PS, Platt RJ, Oricchio E, Reddy ST. Kapetanovic E, et al. Nat Biomed Eng. 2024 Sep 25. doi: 10.1038/s41551-024-01255-x. Online ahead of print. Nat Biomed Eng. 2024. PMID: 39322719 - Adoptive transfer of personalized neoantigen-reactive TCR-transduced T cells in metastatic colorectal cancer: phase 2 trial interim results.
Parkhurst M, Goff SL, Lowery FJ, Beyer RK, Halas H, Robbins PF, Prickett TD, Gartner JJ, Sindiri S, Krishna S, Zacharakis N, Ngo L, Ray S, Bera A, Shepherd R, Levin N, Kim SP, Copeland A, Nah S, Levi S, Parikh N, Kwong MLM, Klemen ND, Yang JC, Rosenberg SA. Parkhurst M, et al. Nat Med. 2024 Sep;30(9):2586-2595. doi: 10.1038/s41591-024-03109-0. Epub 2024 Jul 11. Nat Med. 2024. PMID: 38992129 Clinical Trial. - Identification and validation of tumor-specific T cell receptors from tumor infiltrating lymphocytes using tumor organoid co-cultures.
Li Z, Ma L, Gao Z, Wang X, Che X, Zhang P, Li Y, Zhang Q, Liu T, Sun Y, Bai Y, Deng H. Li Z, et al. Cancer Immunol Immunother. 2024 Jul 2;73(9):164. doi: 10.1007/s00262-024-03749-8. Cancer Immunol Immunother. 2024. PMID: 38954022 Free PMC article.
References
- Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. - PubMed
- Call ME, Wucherpfennig KW. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol. 2005;23:101–25. - PubMed
- Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002;2:512–9. - PubMed
- Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous