Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model - PubMed (original) (raw)
. 2006 Oct;116(10):2663-72.
doi: 10.1172/JCI28773. Epub 2006 Sep 14.
Affiliations
- PMID: 16981011
- PMCID: PMC1564432
- DOI: 10.1172/JCI28773
Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model
Zhigang Yu et al. J Clin Invest. 2006 Oct.
Abstract
Kennedy disease, a degenerative disorder characterized by androgen-dependent neuromuscular weakness, is caused by a CAG/glutamine tract expansion in the androgen receptor (Ar) gene. We developed a mouse model of Kennedy disease, using gene targeting to convert mouse androgen receptor (AR) to human sequence while introducing 113 glutamines. AR113Q mice developed hormone and glutamine length-dependent neuromuscular weakness characterized by the early occurrence of myopathic and neurogenic skeletal muscle pathology and by the late development of neuronal intranuclear inclusions in spinal neurons. AR113Q males unexpectedly died at 2-4 months. We show that this androgen-dependent death reflects decreased expression of skeletal muscle chloride channel 1 (CLCN1) and the skeletal muscle sodium channel alpha-subunit, resulting in myotonic discharges in skeletal muscle of the lower urinary tract. AR113Q limb muscles show similar myopathic features and express decreased levels of mRNAs encoding neurotrophin-4 and glial cell line-derived neurotrophic factor. These data define an important myopathic contribution to the Kennedy disease phenotype and suggest a role for muscle in non-cell autonomous toxicity of lower motor neurons.
Figures
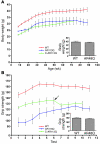
Figure 1. AR113Q males are smaller and weaker than WT littermates.
(A) Body mass (mean ± SD) reported by age of WT (red line, n = 9), AR113Q (blue line, n = 5–22 depending on age, due to early death), and castrated AR113Q males (C-AR113Q) (green line, n = 9). AR113Q males were significantly smaller than WT (P < 0.001), and castration further decreased body mass (_P_ < 0.05 by ANOVA with the Neuman-Keuls multiple comparison test). Inset shows body mass of AR48Q (_n_ = 7) and WT (_n_ = 5) males at 22–23 months (_P_ > 0.05 by unpaired Student’s t test). (B) Forelimb grip strength (mean ± SD) of WT (red line, n = 13), AR113Q (blue line, n = 5), and castrated AR113Q males (green line, n = 9) evaluated monthly beginning at 11–13 months. The 3 curves are significantly different from each other (P < 0.001 by ANOVA with the Neuman-Keuls multiple comparison test). Implantation of testosterone pellets into castrated AR113Q males at 17–19 months is indicated by arrow. Inset shows grip strength of AR48Q (_n_ = 7) and WT (_n_ = 5) males at 22–23 months (_P_ > 0.05 by unpaired Student’s t test).
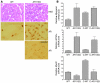
Figure 2. Neuromuscular pathology in AR113Q male mice.
(A) Grouped atrophic, angulated fibers (upper left) and marked variation in fiber size and internally placed nuclei (upper right) are present in hind-limb skeletal muscle of AR113Q males. Magnification, ×400 (upper left); ×1000 (upper right). AR (lower left) and ubiquitin (lower right) immunoreactive intranuclear inclusions are also present. Magnification, ×1000. (B) Relative myogenin, acetylcholine receptor α_-subunit_ (_AChR_α), and MyoD mRNA expression levels in hind-limb muscle as determined by quantitative real-time RT-PCR. Data are from WT (n = 8), AR113 (n = 8), and castrated WT males (C-WT) (n = 6) at 3–5 months and castrated AR113Q males at 18 months (n = 4). Results are reported as mean ± SD relative to expression of 18s rRNA. Expression levels of myogenin and acetylcholine receptor α-subunit mRNA in AR113Q muscle are significantly different from those in all other groups (P < 0.01 and _P_ < 0.001, respectively, by ANOVA with the Neuman-Keuls multiple comparison test). Expression of _MyoD_ mRNA in AR113Q and WT muscle is not significantly different. (**C**) Relative _AR_ mRNA expression levels in hind-limb muscle of WT (_n_ = 8) and AR113Q males (_n_ = 8) at 3–5 months (_P_ > 0.05). (D) AR protein expression in skeletal muscle (top panel) and spinal cord (bottom panel) of WT (lanes 1 and 2) and AR113Q males (lanes 3 and 4) at 3–4 months detected by immunoprecipitation and Western blot. (E) Intranuclear inclusions in spinal cord and skeletal muscle of AR113Q mice at 24 months detected by immunohistochemistry for AR and expanded glutamine tract (1C2). Magnification, ×1000.
Figure 3. Early death in AR113Q males is androgen dependent.
(A) Survival curve of littermate N2 WT (red line, n = 19), AR113Q (blue line, n = 22), and castrated AR113Q males (green line, n = 9). Four of the castrated AR113Q males were euthanized at 18 months (indicated by arrow), and the remaining 5 were implanted with testosterone pellets and monitored for survival. (B) Survival curve of N5 AR113Q males from the original line (black line, n = 14) and N2 AR113Q males from an independently generated second line (blue line, n = 14). (C) Testes of WT, AR48Q, and AR113Q males at 24 months. Magnification, ×400.
Figure 4. Testosterone-treated AR113Q females exhibit normal life span.
(A–D) Testosterone-dependent effects on heterozygous AR113Q females. AR113Q females were implanted with testosterone (+T, n = 8) or vehicle control (–T, n = 7) pellets at 2–4 months. Serum testosterone levels, determined at 4–7 months, were significantly increased in hormone-treated mice compared with controls (P < 0.001) (**A**). Excretion of major urinary proteins (**B**) was compared in AR113Q–T and AR113Q+T females. AR113Q+T females showed a small but significant deficit in forelimb grip strength (**C**) (_P_ = 0.01 by unpaired Student’s _t_ test) but unchanged body mass (**D**) (_P_ = 0.3 by unpaired Student’s _t_ test) compared with AR113Q–T females at 16 months. (**E**) Survival curves of AR113Q+T (_n_ = 8) and AR113Q–T (_n_ = 7) (overlapping green lines), untreated AR113Q females (blue line, _n_ = 11), and WT females (red line, _n_ = 19) were similar (_P_ > 0.05). None of the AR113Q+T or AR113Q–T females died during the study; half were euthanized at 16–19 months, and the remainder were euthanized at 23–26 months.
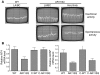
Figure 5. Levator ani/bulbocavernosus muscles exhibit severe pathology in young AR113Q males.
(A) Representative images of levator ani/bulbocavernosus muscle from WT and AR113Q males. Magnification, ×400 (top row). Frequent AR immunoreactive intranuclear inclusions were present in muscle from AR113Q but not WT mice. Magnification, ×1000 (middle row). Muscle from castrated AR113Q males also contained frequent AR immunoreactive intranuclear inclusions. Magnification, ×1000 (bottom row). (B) Altered expression of myogenin and acetylcholine receptor α_-subunit_ mRNA but not MyoD mRNA in AR113Q muscle. Relative mRNA expression levels were determined by quantitative real-time RT-PCR. Data are from WT (n = 7), AR113Q (n = 8), and castrated WT males (n = 6) at 3–5 months and surgically castrated AR113Q males at 18 months (n = 4). Results are reported as mean ± SD relative to expression of 18s rRNA. Differences between WT and AR113Q are significant at P < 0.05 for myogenin and acetylcholine receptor α-subunit mRNA by ANOVA with the Neuman-Keuls multiple comparison test. Expression of MyoD mRNA is not different between WT and AR113Q.
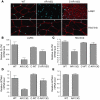
Figure 6. Androgen-dependent decrease of CLCN1 and SCN4A expression in AR113Q skeletal muscle.
(A) Indirect immunofluorescence demonstrates CLCN1 protein expression (red) in levator ani/bulbocavernosus (LA/BC) and hind-limb muscles of WT, AR113Q, and castrated AR113Q males. Nuclei were stained with DAPI. (B–D) Relative CLCN1 (B and C) and SCN4A (D and E) mRNA expression levels in levator ani/bulbocavernosus (B and D) and hind-limb (C and E) muscles of WT (n = 8), AR113Q (n = 9), and castrated WT males (n = 6) at 3–5 months and castrated AR113Q males at 18 months (n = 4). Data are reported as mean ± SD relative to expression of 18s rRNA. Differences between WT and AR113Q are significant different in hind-limb (P < 0.01 for CLCN1) and levator ani/bulbocavernosus muscles (P < 0.001 for CLCN1 and SCN4A) as determined by ANOVA with the Neuman-Keuls multiple comparison test. SCN4A levels in hind-limb muscle are not significantly different.
Figure 7. Electrophysiologic and gene expression changes demonstrate myopathy in AR113Q males.
(A) Composite images from sequential video frames of needle electromyography on levator ani/bulbocavernosus and hind-limb muscles from WT and AR113Q males at 3–5 months. Abnormal spontaneous and insertional activities were observed in both muscle groups of AR113Q males, as indicated by positive, downward deflections of the tracing, but not in WT males. Each horizontal division is equal to 10 ms. (B) Relative NT-4 and GDNF mRNA expression levels in hind-limb muscle, determined as in Figure 6. Differences between WT and AR113Q are significant at P < 0.05.
Similar articles
- Spinal and bulbar muscular atrophy.
Lieberman AP. Lieberman AP. Handb Clin Neurol. 2018;148:625-632. doi: 10.1016/B978-0-444-64076-5.00040-5. Handb Clin Neurol. 2018. PMID: 29478604 Review. - The androgen receptor's CAG/glutamine tract in mouse models of neurological disease and cancer.
Lieberman AP, Robins DM. Lieberman AP, et al. J Alzheimers Dis. 2008 Jun;14(2):247-55. doi: 10.3233/jad-2008-14212. J Alzheimers Dis. 2008. PMID: 18560135 Free PMC article. - Altered RNA splicing contributes to skeletal muscle pathology in Kennedy disease knock-in mice.
Yu Z, Wang AM, Robins DM, Lieberman AP. Yu Z, et al. Dis Model Mech. 2009 Sep-Oct;2(9-10):500-7. doi: 10.1242/dmm.003301. Epub 2009 Aug 19. Dis Model Mech. 2009. PMID: 19692580 Free PMC article. - Loss of endogenous androgen receptor protein accelerates motor neuron degeneration and accentuates androgen insensitivity in a mouse model of X-linked spinal and bulbar muscular atrophy.
Thomas PS Jr, Fraley GS, Damian V, Woodke LB, Zapata F, Sopher BL, Plymate SR, La Spada AR. Thomas PS Jr, et al. Hum Mol Genet. 2006 Jul 15;15(14):2225-38. doi: 10.1093/hmg/ddl148. Epub 2006 Jun 13. Hum Mol Genet. 2006. PMID: 16772330 - Spinal and bulbar muscular atrophy: ligand-dependent pathogenesis and therapeutic perspectives.
Katsuno M, Adachi H, Tanaka F, Sobue G. Katsuno M, et al. J Mol Med (Berl). 2004 May;82(5):298-307. doi: 10.1007/s00109-004-0530-7. Epub 2004 Feb 27. J Mol Med (Berl). 2004. PMID: 15133611 Review.
Cited by
- Spinal and bulbar muscular atrophy: pathogenesis and clinical management.
Grunseich C, Rinaldi C, Fischbeck KH. Grunseich C, et al. Oral Dis. 2014 Jan;20(1):6-9. doi: 10.1111/odi.12121. Epub 2013 May 9. Oral Dis. 2014. PMID: 23656576 Free PMC article. Review. - Neuromuscular junctions are pathological but not denervated in two mouse models of spinal bulbar muscular atrophy.
Poort JE, Rheuben MB, Breedlove SM, Jordan CL. Poort JE, et al. Hum Mol Genet. 2016 Sep 1;25(17):3768-3783. doi: 10.1093/hmg/ddw222. Epub 2016 Aug 4. Hum Mol Genet. 2016. PMID: 27493028 Free PMC article. - Disease Affects Bdnf Expression in Synaptic and Extrasynaptic Regions of Skeletal Muscle of Three SBMA Mouse Models.
Halievski K, Nath SR, Katsuno M, Adachi H, Sobue G, Breedlove SM, Lieberman AP, Jordan CL. Halievski K, et al. Int J Mol Sci. 2019 Mar 15;20(6):1314. doi: 10.3390/ijms20061314. Int J Mol Sci. 2019. PMID: 30875922 Free PMC article. - Genetic approaches to the treatment of inherited neuromuscular diseases.
Ravi B, Antonellis A, Sumner CJ, Lieberman AP. Ravi B, et al. Hum Mol Genet. 2019 Oct 1;28(R1):R55-R64. doi: 10.1093/hmg/ddz131. Hum Mol Genet. 2019. PMID: 31227836 Free PMC article. Review. - Gene therapy with AR isoform 2 rescues spinal and bulbar muscular atrophy phenotype by modulating AR transcriptional activity.
Lim WF, Forouhan M, Roberts TC, Dabney J, Ellerington R, Speciale AA, Manzano R, Lieto M, Sangha G, Banerjee S, Conceição M, Cravo L, Biscans A, Roux L, Pourshafie N, Grunseich C, Duguez S, Khvorova A, Pennuto M, Cortes CJ, La Spada AR, Fischbeck KH, Wood MJA, Rinaldi C. Lim WF, et al. Sci Adv. 2021 Aug 20;7(34):eabi6896. doi: 10.1126/sciadv.abi6896. Print 2021 Aug. Sci Adv. 2021. PMID: 34417184 Free PMC article.
References
- Zoghbi H.Y., Orr H.T. Glutamine repeats and neurode generation. Annu. Rev. Neurosci. 2000;23:217–247. - PubMed
- Lieberman A.P., Fischbeck K.H. Triplet repeat expansion in neuromuscular disease. Muscle Nerve. 2000;23:843–850. - PubMed
- Katsuno M., et al. Pathogenesis, animal models and therapeutics in Spinal and bulbar muscular atrophy (SBMA). Exp. Neurol. 2006;200:8–18. - PubMed
- Sperfeld A.D., et al. X-linked bulbospinal neuronopathy: Kennedy disease. Arch. Neurol. 2002;59:1921–1926. - PubMed
- Abel A., Walcott J., Woods J., Duda J., Merry D.E. Expression of expanded repeat androgen receptor produces neurologic disease in transgenic mice. Hum. Mol. Genet. 2001;10:107–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials