Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord - PubMed (original) (raw)
Comparative Study
Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord
Laura Taylor et al. J Neurosci. 2006.
Abstract
Neurotrophic factor delivery to sites of spinal cord injury (SCI) promotes axon growth into but not beyond lesion sites. We tested the hypothesis that sustained growth factor gradients beyond regions of SCI will promote significant axonal bridging into and beyond lesions. Adult rats underwent C3 lesions to transect ascending dorsal column sensory axons, and autologous bone marrow stromal cells were grafted into the lesion to provide a cellular bridge for growth into the injured region. Concurrently, lentiviral vectors expressing neurotrophin-3 (NT-3) or green fluorescent protein (GFP) (controls) were injected into the host cord rostral to the lesion to promote axon extension beyond the graft/lesion. Four weeks later, NT-3 gradients beyond the lesion were detectable by ELISA in animals that received NT-3-expressing lentiviral vectors, with highest average NT-3 levels located near the rostral vector injection site. Significantly more ascending sensory axons extended into tissue rostral to the lesion site in animals injected with NT-3 vectors compared with GFP vectors, but only if the zone of NT-3 vector transduction extended continuously from the injection site to the graft; any "gap" in NT-3 expression from the graft to rostral tissue resulted in axon bridging failure. Despite axon bridging beyond the lesion, regenerating axons did not continue to grow over very long distances, even in the presence of a continuing growth factor gradient beyond the lesion. These findings indicate that a localized and continuous gradient of NT-3 can achieve axonal bridging beyond the glial scar, but growth for longer distances is not sustainable simply with a trophic stimulus.
Figures
Figure 1.
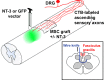
Schematic illustrating experimental design. Adult rats underwent dorsal column wire knife lesions at C3 to transect ascending sensory axons (inset). Autologous bone marrow stromal cells (either naive or modified in vitro to express NT-3) were grafted into the lesion site, and lentiviral vectors expressing NT-3 or GFP (control) were injected into the spinal cord 2.5 mm rostral to the lesion site. After 4 weeks, animals were killed for ELISA or ascending sensory axons were traced by injection of CTB into the sciatic nerve, and animals were killed 3 d later for histology.
Figure 2.
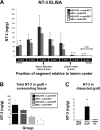
Injection of NT-3-expressing lentiviral vectors establishes a gradient of NT-3 in the injured spinal cord. A, Four weeks after lesion and vector injection, spinal cords were sectioned into 1 mm segments as shown schematically on the _x_-axis. ELISA of individual 1 mm segments indicated that, in animals that received Lenti–NT-3 vector injections but not Lenti–GFP injections, a gradient of NT-3 was established rostral to the lesion site. For groups that received Lenti–NT-3 vector injections, asterisks indicate differences in NT-3 levels between the vector injection site (3 mm segment) and other segments in the same treatment group (p < 0.001, repeated-measures ANOVA; *p < 0.05, **p < 0.01, Fisher's post hoc test). Values are mean ± SEM. B, Total NT-3 levels in the two spinal cord segments containing the graft/lesion site, and surrounding host tissue (1 and −1 mm), were greatly increased in animals that received Lenti–NT-3 vectors compared with Lenti–GFP vectors. In animals that received Lenti–GFP vectors, expression of NT-3 by genetically modified MSC grafts was evident as a twofold increase in total NT-3 detected in the 1 and −1 mm spinal cord segments. Note that these measures include all host tissue within the spinal cord surrounding the lesion site, diluting amounts of NT-3 produced by NT-3-secreting MSCs in the lesion site. C, Levels of NT-3 were also measured in grafts specifically dissected from the lesion site, undiluted by surrounding host tissue. ELISA on this specific MSC graft tissue showed significantly higher levels of NT-3 in MSC–NT-3 grafted animals compared with MSC grafted animals or intact spinal cord (p < 0.01, ANOVA; *p < 0.05, Fisher's post hoc test).
Figure 3.
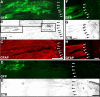
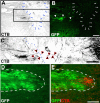
Ascending sensory axons extend beyond the graft/lesion site toward NT-3 vector-transduced cells. Triple immunolabeling for GFP to label NT-3 vector-transduced cells (A), GFAP to indicate the extent of the lesion/graft site (B), and CTB to label ascending sensory axons (C) in a sagittally cut spinal cord 4 weeks after MSC–NT-3 cell grafting and Lenti–NT-3 injection. A′–C′, Higher magnification of the rostral host/graft interface shows axons (C′; arrows) growing into GFAP-rich regions beyond the lesion site (B′) in which vector-transduced cells express NT-3 (A′). D, E, High magnification of red and blue boxed areas in C and C′, respectively. F, G, Higher magnification of boxed regions in D and E demonstrate the association of CTB-labeled axons (pseudocolored purple) with Lenti–NT-3 vector-transduced cells expressing the GFP reporter (green). Arrows in A–C and dashed lines in A′–C′ indicate the graft/astrocyte border. Rostral is to the left, and dorsal to the top. Scale bars: A–C, 200 μm; A′–C′, 100 μm; D, E, 50 μm; F, G, 10 μm.
Figure 4.
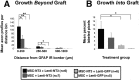
Quantification of CTB-labeled axonal profiles rostral to and within the lesion. Animals that received MSC–NT-3 graft cells combined with Lenti–NT-3 injections exhibited significantly more axonal profiles beyond the astrocyte-defined lesion border than animals from any other group (A) and significantly higher axonal density within the lesion border than animals that received Lenti–GFP injections (B) (ANOVA; *p < 0.05, Fisher's post hoc analysis). Values are mean ± SEM. IR, Immunoreactivity. ** p < 0.01.
Figure 5.
Axonal growth beyond the lesion site occurred only when lentivirus-mediated NT-3 expression was located within 100 μm of the lesion border. Triple immunolabeling for GFP (A), CTB (B), and GFAP (C) shows that, in subjects exhibiting bridging axons, Lenti–NT-3-transduced cells (expressing the reporter GFP) were evident within 100 μm of the host/lesion border. Boxes in B indicate regions of high axon density rostral to the lesion site (Lenti–NT-3/MSC treatment group). When the zone of Lenti–NT-3 transduction was distant from the lesion site (D), bridging axons were not observed (E) (Lenti–NT-3/MSC treatment group). F–H, In animals injected with Lenti–GFP, axons did not extend beyond the graft, regardless of the distance between Lenti–GFP transduction and the lesion site (Lenti–GFP/MSC–NT-3 treatment group). Rostral is to the left, and dorsal to the top. Scale bars: A–C, 140 μm; D, E, 210 μm; F–H, 100 μm.
Figure 6.
The topography of sensory axon bridging beyond the lesion site corresponds to the location of NT-3 lentiviral transduction. A, CTB-labeled axons (box) crossing the lesion extend beyond the lesion border (arrows), toward the region of dorsal column white matter in which cells have been transduced by Lenti–NT-3 vectors (B; arrowheads). C, Axons can be traced from the main CTB-labeled tract at the caudal end of the lesion site, across the lesion site, and beyond the graft/host interface (dotted lines). D, On reaching areas of high NT-3 expression distal to a lesion site (outlined by dashed lines), axons (E; pseudocolored red) sprout locally. These axons do not extend for long distances within NT-3-expressing tissue. Scale bars: A, B, 200 μm; C, 140 μm; E, F, 100 μm.
Similar articles
- Dependence of regenerated sensory axons on continuous neurotrophin-3 delivery.
Hou S, Nicholson L, van Niekerk E, Motsch M, Blesch A. Hou S, et al. J Neurosci. 2012 Sep 19;32(38):13206-20. doi: 10.1523/JNEUROSCI.5041-11.2012. J Neurosci. 2012. PMID: 22993437 Free PMC article. - Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds.
Gros T, Sakamoto JS, Blesch A, Havton LA, Tuszynski MH. Gros T, et al. Biomaterials. 2010 Sep;31(26):6719-29. doi: 10.1016/j.biomaterials.2010.04.035. Epub 2010 Jun 17. Biomaterials. 2010. PMID: 20619785 - Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury.
Liu S, Sandner B, Schackel T, Nicholson L, Chtarto A, Tenenbaum L, Puttagunta R, Müller R, Weidner N, Blesch A. Liu S, et al. Acta Biomater. 2017 Sep 15;60:167-180. doi: 10.1016/j.actbio.2017.07.024. Epub 2017 Jul 19. Acta Biomater. 2017. PMID: 28735026 - Determining Neurotrophin Gradients in Vitro To Direct Axonal Outgrowth Following Spinal Cord Injury.
Dravid A, Parittotokkaporn S, Aqrawe Z, O'Carroll SJ, Svirskis D. Dravid A, et al. ACS Chem Neurosci. 2020 Jan 15;11(2):121-132. doi: 10.1021/acschemneuro.9b00565. Epub 2019 Dec 26. ACS Chem Neurosci. 2020. PMID: 31825204 Review. - Gene therapy approaches for neuroprotection and axonal regeneration after spinal cord and spinal root injury.
Bo X, Wu D, Yeh J, Zhang Y. Bo X, et al. Curr Gene Ther. 2011 Apr;11(2):101-15. doi: 10.2174/156652311794940773. Curr Gene Ther. 2011. PMID: 21291358 Review.
Cited by
- NT-3 promotes proprioceptive axon regeneration when combined with activation of the mTor intrinsic growth pathway but not with reduction of myelin extrinsic inhibitors.
Liu Y, Kelamangalath L, Kim H, Han SB, Tang X, Zhai J, Hong JW, Lin S, Son YJ, Smith GM. Liu Y, et al. Exp Neurol. 2016 Sep;283(Pt A):73-84. doi: 10.1016/j.expneurol.2016.05.021. Epub 2016 Jun 2. Exp Neurol. 2016. PMID: 27264357 Free PMC article. - Neurotrophic factors in combinatorial approaches for spinal cord regeneration.
McCall J, Weidner N, Blesch A. McCall J, et al. Cell Tissue Res. 2012 Jul;349(1):27-37. doi: 10.1007/s00441-012-1388-6. Epub 2012 Apr 12. Cell Tissue Res. 2012. PMID: 22526621 Free PMC article. Review. - Ex Vivo Rat Transected Spinal Cord Slices as a Model to Assess Lentiviral Vector Delivery of Neurotrophin-3 and Short Hairpin RNA against NG2.
Patar A, Dockery P, McMahon S, Howard L. Patar A, et al. Biology (Basel). 2020 Mar 15;9(3):54. doi: 10.3390/biology9030054. Biology (Basel). 2020. PMID: 32183469 Free PMC article. - Low-density lipoprotein receptor-related protein 1 (LRP1)-dependent cell signaling promotes axonal regeneration.
Yoon C, Van Niekerk EA, Henry K, Ishikawa T, Orita S, Tuszynski MH, Campana WM. Yoon C, et al. J Biol Chem. 2013 Sep 13;288(37):26557-68. doi: 10.1074/jbc.M113.478552. Epub 2013 Jul 18. J Biol Chem. 2013. PMID: 23867460 Free PMC article. - Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp-mediated effects.
Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, Geschwind D, Tuszynski MH. Blesch A, et al. Exp Neurol. 2012 May;235(1):162-73. doi: 10.1016/j.expneurol.2011.12.037. Epub 2011 Dec 29. Exp Neurol. 2012. PMID: 22227059 Free PMC article.
References
- Baekelandt V, Claeys A, Eggermont K, Lauwers E, De Strooper B, Nuttin B, Debyser Z. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum Gene Ther. 2002;13:841–853. - PubMed
- Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur J Neurosci. 2001;13:257–268. - PubMed
- Blesch A. Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods. 2004;33:164–172. - PubMed
- Blesch A, Yang H, Weidner N, Hoang A, Otero D. Axonal responses to cellularly delivered NT-4/5 after spinal cord injury. Mol Cell Neurosci. 2004;27:190–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous