Rescue of TRAF3-null mice by p100 NF-kappa B deficiency - PubMed (original) (raw)
Rescue of TRAF3-null mice by p100 NF-kappa B deficiency
Jeannie Q He et al. J Exp Med. 2006.
Abstract
Proper activation of nuclear factor (NF)-kappaB transcription factors is critical in regulating fundamental biological processes such as cell survival and proliferation, as well as in inflammatory and immune responses. Recently, the NF-kappaB signaling pathways have been categorized into the canonical pathway, which results in the nuclear translocation of NF-kappaB complexes containing p50, and the noncanonical pathway, which involves the induced processing of p100 to p52 and the formation of NF-kappaB complexes containing p52 (Bonizzi, G., and M. Karin. 2004. Trends Immunol. 25:280-288). We demonstrate that loss of tumor necrosis factor (TNF) receptor-associated factor 3 (TRAF3) results in constitutive noncanonical NF-kappaB activity. Importantly, TRAF3-/- B cells show ligand-independent up-regulation of intracellular adhesion molecule 1 and protection from spontaneous apoptosis during in vitro culture. In addition, we demonstrate that loss of TRAF3 results in profound accumulation of NF-kappaB-inducing kinase in TRAF3-/- cells. Finally, we show that the early postnatal lethality observed in TRAF3-deficient mice is rescued by compound loss of the noncanonical NF-kappaB p100 gene. Thus, these genetic data clearly demonstrate that TRAF3 is a critical negative modulator of the noncanonical NF-kappaB pathway and that constitutive activation of the noncanonical NF-kappaB pathway causes the lethal phenotype of TRAF3-deficient mice.
Figures
Figure 1.
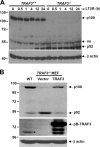
TRAF3 deficiency results in constitutive processing of p100 in MEFs. (A) WT and TRAF3 −_/− MEFs were stimulated with an anti-LTβR antibody for the indicated times. Processing of p100 to p52 in whole cell extract was detected by immunoblot. Total β actin is shown as a loading control. (B) Whole cell extracts from WT and TRAF3 −/− MEFs reconstituted with either pBABEpuro-TAP or pBABEpuro-TAP-TRAF3 were assessed for basal p100 processing to p52 by immunoblot. Reconstitution of TRAF3 expression in TRAF3 −/_− MEFs was confirmed by immunoblot. Total β actin is shown as a loading control. *, endogenous TRAF3.
Figure 2.
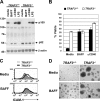
TRAF3-deficient B lymphocytes display ligand-independent activation. (A) WT and TRAF3 −_/− B cells were stimulated with BAFF, anti-CD40 antibody, or LPS for 24 h, and processing of p100 to p52 was detected by immunoblot. β actin is shown as a loading control. (B) WT and TRAF3 −/− B cells were stimulated with BAFF or anti-CD40 antibody for 96 h, and cell death was measured by staining with propidium iodide. Error bars indicate ±1 SD between duplicate samples. WT and TRAF3 −/_− B cells were stimulated with BAFF for 48 h, and (C) analysis of ICAM-1 protein expression was determined by FACS and (D) images of cells forming homotypic aggregates were taken (see In vitro B cell assays for details).
Figure 3.
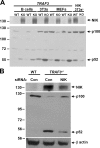
Enhanced NIK expression levels in TRAF3-deficient cells. (A) 150 μg of whole cell extracts from v-ABL–transformed WT and TRAF3 −_/− B cells, 3T3s, and primary MEFs were assessed by immunoblotting for NIK. p100 processing to p52 in these cells was confirmed by immunoblot. *, NIK +/+ and NIK −/− 3T3s were treated with MG132 for 2 h to serve as a control. (B) WT and TRAF3 −/_− MEFs were transfected with 150 nM control or NIK-siRNA. Whole cell extracts were obtained 48 h after transfection, and NIK and p100/p52 levels were detected by immunoblot. β actin is shown as a loading control.
Figure 4.
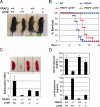
Rescue of TRAF3-null phenotypes by combined deletion of the p100 gene. The rescue of the TRAF3-null phenotype was examined by body size (A), survival (B), spleen size and total splenocyte count (C; error bars indicate ±1 SD between three mice), and serum glucose and corticosterone concentration (D; error bars indicate ±1 SD between two and five mice).
Similar articles
- Specificity of TRAF3 in its negative regulation of the noncanonical NF-kappa B pathway.
He JQ, Saha SK, Kang JR, Zarnegar B, Cheng G. He JQ, et al. J Biol Chem. 2007 Feb 9;282(6):3688-94. doi: 10.1074/jbc.M610271200. Epub 2006 Dec 11. J Biol Chem. 2007. PMID: 17158868 - NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism.
Yao Z, Xing L, Boyce BF. Yao Z, et al. J Clin Invest. 2009 Oct;119(10):3024-34. doi: 10.1172/JCI38716. Epub 2009 Sep 21. J Clin Invest. 2009. PMID: 19770515 Free PMC article. - Control of canonical NF-kappaB activation through the NIK-IKK complex pathway.
Zarnegar B, Yamazaki S, He JQ, Cheng G. Zarnegar B, et al. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3503-8. doi: 10.1073/pnas.0707959105. Epub 2008 Feb 21. Proc Natl Acad Sci U S A. 2008. PMID: 18292232 Free PMC article. - The noncanonical NF-κB pathway.
Sun SC. Sun SC. Immunol Rev. 2012 Mar;246(1):125-40. doi: 10.1111/j.1600-065X.2011.01088.x. Immunol Rev. 2012. PMID: 22435551 Free PMC article. Review. - TRAF3 and its biological function.
He JQ, Oganesyan G, Saha SK, Zarnegar B, Cheng G. He JQ, et al. Adv Exp Med Biol. 2007;597:48-59. doi: 10.1007/978-0-387-70630-6_4. Adv Exp Med Biol. 2007. PMID: 17633016 Review.
Cited by
- The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity.
Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z, Shaheen ZR, Cheng G, Sawatzke K, Campbell AM, Auger JL, Bilgic H, Shoyama FM, Schmeling DO, Balfour HH Jr, Hasegawa K, Chan AC, Corbett JA, Binstadt BA, Mescher MF, Ley K, Bottini N, Peterson EJ. Wang Y, et al. Immunity. 2013 Jul 25;39(1):111-22. doi: 10.1016/j.immuni.2013.06.013. Epub 2013 Jul 18. Immunity. 2013. PMID: 23871208 Free PMC article. - Return to homeostasis: downregulation of NF-κB responses.
Ruland J. Ruland J. Nat Immunol. 2011 Jun 19;12(8):709-14. doi: 10.1038/ni.2055. Nat Immunol. 2011. PMID: 21772279 Review. - Hodgkin lymphoma requires stabilized NIK and constitutive RelB expression for survival.
Ranuncolo SM, Pittaluga S, Evbuomwan MO, Jaffe ES, Lewis BA. Ranuncolo SM, et al. Blood. 2012 Nov 1;120(18):3756-63. doi: 10.1182/blood-2012-01-405951. Epub 2012 Sep 11. Blood. 2012. PMID: 22968463 Free PMC article. - TRAF3 Modulation: Novel Mechanism for the Anti-inflammatory Effects of the Vitamin D Receptor Agonist Paricalcitol in Renal Disease.
Rayego-Mateos S, Morgado-Pascual JL, Valdivielso JM, Sanz AB, Bosch-Panadero E, Rodrigues-Díez RR, Egido J, Ortiz A, González-Parra E, Ruiz-Ortega M. Rayego-Mateos S, et al. J Am Soc Nephrol. 2020 Sep;31(9):2026-2042. doi: 10.1681/ASN.2019111206. Epub 2020 Jul 6. J Am Soc Nephrol. 2020. PMID: 32631974 Free PMC article. - Non-canonical NF-κB signaling pathway.
Sun SC. Sun SC. Cell Res. 2011 Jan;21(1):71-85. doi: 10.1038/cr.2010.177. Epub 2010 Dec 21. Cell Res. 2011. PMID: 21173796 Free PMC article. Review.
References
- Bonizzi, G., and M. Karin. 2004. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280–288. - PubMed
- Hayden, M.S., and S. Ghosh. 2004. Signaling to NF-kappaB. Genes Dev. 18:2195–2224. - PubMed
- Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 18:621–663. - PubMed
- Xiao, G., E.W. Harhaj, and S.C. Sun. 2001. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell. 7:401–409. - PubMed
- Dejardin, E., N.M. Droin, M. Delhase, E. Haas, Y. Cao, C. Makris, Z.W. Li, M. Karin, C.F. Ware, and D.R. Green. 2002. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 17:525–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM57559/GM/NIGMS NIH HHS/United States
- T32 GM008042/GM/NIGMS NIH HHS/United States
- T32 AI007126/AI/NIAID NIH HHS/United States
- R01 CA087924/CA/NCI NIH HHS/United States
- GM 08042/GM/NIGMS NIH HHS/United States
- AI07126-30/AI/NIAID NIH HHS/United States
- R01 AI056154/AI/NIAID NIH HHS/United States
- R01 CA87924/CA/NCI NIH HHS/United States
- R01 GM057559/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials