A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas - PubMed (original) (raw)
Clinical Trial
. 2006 Nov 6;95(9):1148-54.
doi: 10.1038/sj.bjc.6603419. Epub 2006 Oct 10.
J Kortmansky, D Singh, H Hirte, W Kocha, G Goss, L Le, A Oza, T Nicklee, J Ho, D Birle, G R Pond, D Arboine, J Dancey, S Aviel-Ronen, M-S Tsao, D Hedley, L L Siu
Affiliations
- PMID: 17031397
- PMCID: PMC2360568
- DOI: 10.1038/sj.bjc.6603419
Clinical Trial
A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas
I Duran et al. Br J Cancer. 2006.
Abstract
Standard cytotoxic treatments for neuroendocrine tumours have been associated with limited activity and remarkable toxicity. A phase II study was designed to evaluate the efficacy, safety and pharmacodynamics of temsirolimus in patients with advanced neuroendocrine carcinoma (NEC). Thirty-seven patients with advanced progressive NEC received intravenous weekly doses of 25 mg of temsirolimus. Patients were evaluated for tumour response, time to progression (TTP), overall survival (OS) and adverse events (AE). Twenty-two archival specimens, as well as 13 paired tumour biopsies obtained pretreatment and after 2 weeks of temsirolimus were assessed for potential predictive and correlative markers. The intent-to-treat response rate was 5.6% (95% CI 0.6-18.7%), median TTP 6 months and 1-year OS rate 71.5%. The most frequent drug-related AE of all grades as percentage of patients were: fatigue (78%), hyperglycaemia (69%) and rash/desquamation (64%). Temsirolimus effectively inhibited the phosphorylation of S6 (P=0.02). Higher baseline levels of pmTOR (phosphorylated mammalian target of rapamycin) (P=0.01) predicted for a better response. Increases in pAKT (P=0.041) and decreases in pmTOR (P=0.048) after treatment were associated with an increased TTP. Temsirolimus appears to have little activity and does not warrant further single-agent evaluation in advanced NEC. Pharmacodynamic analysis revealed effective mTOR pathway downregulation.
Figures
Figure 1
Maximal percentages of tumour reduction for target lesion(s) by RECIST criteria (Note: some patients with PD progressed owing to new or increasing non-target lesions, or by symptomatic progression).
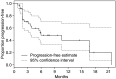
Figure 2
Time to progression for entire study cohort.
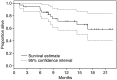
Figure 3
Overall survival for entire study cohort.
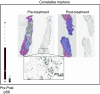
Figure 4
Pre- and post-treatment liver biopsies. Tissue sections were first immunofluorescence-labelled for S235/236-S6 ribosomal protein, imaged, and then restained with H&E. The grey scale images of pS6 are unenhanced, at original resolution.
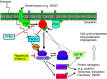
Figure 5
PI3K/AKT/mTOR pathway showing the mTOR protein complexes, mTOR/RAPTOR and mTOR/RICTOR, and the feedback loop involving IGF-IR. Arrows indicate activation; bars indicate inhibition.
Comment in
- Evaluating the activity of temsirolimus in neuroendocrine cancer.
O'Donnell PH, Ratain MJ. O'Donnell PH, et al. Br J Cancer. 2007 Jan 15;96(1):177; author reply 178-9. doi: 10.1038/sj.bjc.6603513. Epub 2006 Dec 12. Br J Cancer. 2007. PMID: 17164757 Free PMC article. No abstract available.
Similar articles
- Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies.
Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, Reddy SA, Davis D, Zhang Y, Wolff RA, Abbruzzese JL. Javle MM, et al. BMC Cancer. 2010 Jul 14;10:368. doi: 10.1186/1471-2407-10-368. BMC Cancer. 2010. PMID: 20630061 Free PMC article. Clinical Trial. - Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors.
Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J, Berkenblit A, Krygowski M, Ananthakrishnan R, Boni JP, Gilbertson RJ. Spunt SL, et al. J Clin Oncol. 2011 Jul 20;29(21):2933-40. doi: 10.1200/JCO.2010.33.4649. Epub 2011 Jun 20. J Clin Oncol. 2011. PMID: 21690471 Free PMC article. Clinical Trial. - Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma.
Cho D, Signoretti S, Dabora S, Regan M, Seeley A, Mariotti M, Youmans A, Polivy A, Mandato L, McDermott D, Stanbridge E, Atkins M. Cho D, et al. Clin Genitourin Cancer. 2007 Sep;5(6):379-85. doi: 10.3816/CGC.2007.n.020. Clin Genitourin Cancer. 2007. PMID: 17956710 - Temsirolimus.
Schulze M, Stock C, Zaccagnini M, Teber D, Rassweiler JJ. Schulze M, et al. Recent Results Cancer Res. 2014;201:393-403. doi: 10.1007/978-3-642-54490-3_24. Recent Results Cancer Res. 2014. PMID: 24756806 Review. - Temsirolimus, an mTOR inhibitor for treatment of patients with advanced renal cell carcinoma.
Malizzia LJ, Hsu A. Malizzia LJ, et al. Clin J Oncol Nurs. 2008 Aug;12(4):639-46. doi: 10.1188/08.CJON.639-646. Clin J Oncol Nurs. 2008. PMID: 18676330 Review.
Cited by
- Rapamycin selectively reduces the association of transcripts containing complex 5' UTRs with ribosomes in C4-2B prostate cancer cells.
Opdenaker LM, Farach-Carson MC. Opdenaker LM, et al. J Cell Biochem. 2009 Jun 1;107(3):473-81. doi: 10.1002/jcb.22145. J Cell Biochem. 2009. PMID: 19347904 Free PMC article. - PI3K-AKT-mTOR-signaling and beyond: the complex network in gastroenteropancreatic neuroendocrine neoplasms.
Briest F, Grabowski P. Briest F, et al. Theranostics. 2014 Jan 29;4(4):336-65. doi: 10.7150/thno.7851. eCollection 2014. Theranostics. 2014. PMID: 24578720 Free PMC article. Review. - Response to mTOR inhibition: activity of eIF4E predicts sensitivity in cell lines and acquired changes in eIF4E regulation in breast cancer.
Satheesha S, Cookson VJ, Coleman LJ, Ingram N, Madhok B, Hanby AM, Suleman CA, Sabine VS, Macaskill EJ, Bartlett JM, Dixon JM, McElwaine JN, Hughes TA. Satheesha S, et al. Mol Cancer. 2011 Feb 14;10:19. doi: 10.1186/1476-4598-10-19. Mol Cancer. 2011. PMID: 21320304 Free PMC article. - Progress in the treatment of neuroendocrine tumors.
Chan JA, Kulke MH. Chan JA, et al. Curr Oncol Rep. 2009 May;11(3):193-9. doi: 10.1007/s11912-009-0028-0. Curr Oncol Rep. 2009. PMID: 19336011 Free PMC article. Review. - Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives.
Ong PS, Wang LZ, Dai X, Tseng SH, Loo SJ, Sethi G. Ong PS, et al. Front Pharmacol. 2016 Oct 25;7:395. doi: 10.3389/fphar.2016.00395. eCollection 2016. Front Pharmacol. 2016. PMID: 27826244 Free PMC article. Review.
References
- Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, Dukart G, Sherman ML (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22: 909–918 - PubMed
- Baselga J, Fumoleau P, Gil M, Colomer R, Roche H, Cortes-Funes H, Burstein H, Kaufman P, Kong S, Moore L (2004) Phase II, 3-arm study of CCI-779 in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer: preliminary results. Proc Am Soc Clin Oncol 23: 13
- Bukowski RM, Tangen CM, Peterson RF, Taylor SA, Rinehart JJ, Eyre HJ, Rivkin SE, Fleming TR, Macdonald JS (1994) Phase II trial of dimethyltriazenoimidazole carboxamide in patients with metastatic carcinoid. A Southwest Oncology Group study. Cancer 73: 1505–1508 - PubMed
- Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, Ostapenko V, Illiger HJ, Behringer D, Bardy-Bouxin N, Boni J, Kong S, Cincotta M, Moore L (2005) Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 23: 5314–5322 - PubMed
- Cheng PN, Saltz LB (1999) Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer 86: 944–948 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous