Complement activation in the Parkinson's disease substantia nigra: an immunocytochemical study - PubMed (original) (raw)
Complement activation in the Parkinson's disease substantia nigra: an immunocytochemical study
David A Loeffler et al. J Neuroinflammation. 2006.
Abstract
Background: Inflammatory processes are increased in the Parkinson's disease (PD) brain. The long-term use of nonsteroidal anti-inflammatory drugs has been associated, in retrospective studies, with decreased risk for PD, suggesting that inflammation may contribute to development of this disorder. The objective of this study was to determine the extent of complement activation, a major inflammatory mechanism, in PD.
Methods: Substantia nigra specimens from young normal subjects (n = 11-13), aged normal subjects (n = 24-28), and subjects with PD (n = 19-20), Alzheimer's disease (AD; n = 12-13), and dementia with Lewy bodies (DLB; n = 9) were stained for iC3b and C9, representing early- and late-stage complement activation, respectively. Numbers of iC3b+, C9+, and total melanized neurons in each section were counted in a blinded fashion. Nonparametric analyses were used to evaluate differences between groups and to evaluate correlations between complement staining, numbers of melanized neurons, and the duration of PD.
Results: Lewy bodies in both PD and DLB specimens stained for iC3b and C9. Staining was also prominent on melanized neurons. The percentage of iC3b+ neurons was significantly increased in PD vs. aged normal and AD specimens, and in young normal vs. aged normal specimens. C9 immunoreactivity was significantly increased in PD vs. AD specimens, but unlike iC3b, the increased C9 staining in PD and young normal specimens did not achieve statistical significance vs. aged normal specimens. iC3b and C9 staining in PD specimens was not correlated with the numbers of remaining melanized neurons, nor with the duration of PD.
Conclusion: Complement activation occurs on Lewy bodies and melanized neurons in the PD substantia nigra. Early complement activation (iC3b) is increased on melanized neurons in PD vs. aged normal specimens, and late-stage complement activation (C9) also tends to increase. This latter finding suggests that complement activation may contribute to loss of dopaminergic neurons in some individuals with PD. Complement activation on melanized neurons appears to decrease with normal aging, suggesting a possible neuroprotective role for this process in the normal substantia nigra.
Figures
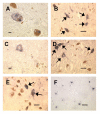
Figure 1
iC3b staining in substantia nigra specimens. Fig. 1A: Immunoreactive Lewy bodies in a PD substantia nigra specimen; Fig. 1B: Staining of melanized neurons (arrows) in a different PD specimen; Fig. 1C: Immunoreactive neuron with little melanin remaining, same PD specimen as Fig. 1B; Fig. 1D: iC3b staining of melanized neurons (arrows) in a young normal specimen; compare with unstained neurons in lower part of field; Fig. 1E: similar staining pattern in an AD specimen; two prominently stained melanized neurons are seen (arrows) among several unstained neurons; Fig. 1F: iC3b-stained senile plaques in a different AD substantia nigra specimen. (Figs. 1A and 1C, bar = 10 μm; Figs. 1B and 1D–F, bar = 50 μm; immunoreactive structures are dark blue or gray, in contrast to brown melanin and yellow background).
Figure 2
C9 staining in substantia nigra specimens. Fig. 2A: Staining of multiple Lewy bodies within a melanized neuron in a PD specimen; adjacent melanized neuron (arrow) and its axon are also C9-positive; Fig. 2B: immunoreactivity for C9 in a Lewy body (arrowhead) and in melanin-depleted neurons (arrows) in a different PD specimen; Fig. 2C: staining of melanized neuron (arrow) and its processes in a DLB specimen; Fig 2D: multiple immunoreactive melanized neurons in an aged normal specimen. (Fig. 2A, bar = 10 μm; Figs. 2B–D, bar = 50 μm; immunoreactive structures are dark blue or gray, in contrast to brown melanin and yellow background).
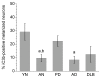
Figure 3
Percentages of iC3b-positive melanized neurons in different groups of substantia nigra specimens. The percentage of iC3b+ melanized neurons was significantly increased in PD vs. both aged normal and AD specimens, and in young normal vs. aged normal specimens. Data are expressed as means ± SEM. (a_p_ < 0.05 vs. PD; b_p_ < 0.05 vs. young normal specimens; abbreviations: AD, Alzheimer's disease; AN, aged normal; DLB, dementia with Lewy bodies; PD, Parkinson's disease; YN, young normal)
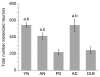
Figure 4
Numbers of melanized neurons in different groups of substantia nigra specimens. Total numbers of melanized neurons were significantly decreased in PD vs. aged normal, young normal, and AD specimens, and in DLB vs. aged normal and AD specimens. Data (means ± SEM) are shown for slides from specimens in which iC3b immunoreactivity was assessed; essentially similar results were obtained for slides from specimens in which C9 staining was evaluated. (a_p_ < 0.05 vs. PD; b_p_ < 0.05 vs. DLB; abbreviations: AD, Alzheimer's disease; AN, aged normal; DLB, dementia with Lewy bodies; PD, Parkinson's disease; YN, young normal)
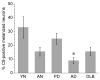
Figure 5
Percentages of C9-positive melanized neurons in different groups of substantia nigra specimens. C9 staining was significantly increased in PD vs. AD specimens. The percentages of C9+ melanized neurons in PD and young normal specimens tended to be increased vs. aged normal specimens, but these differences were not significant (p = 0.04 [not significant after adjustment for multiple comparisons] and 0.08, respectively). Data are expressed as means ± SEM. (a_p_ < 0.05 vs. PD; abbreviations: AD, Alzheimer's disease; AN, aged normal; DLB, dementia with Lewy bodies; PD, Parkinson's disease; YN, young normal)
Similar articles
- Plaque complement activation and cognitive loss in Alzheimer's disease.
Loeffler DA, Camp DM, Bennett DA. Loeffler DA, et al. J Neuroinflammation. 2008 Mar 11;5:9. doi: 10.1186/1742-2094-5-9. J Neuroinflammation. 2008. PMID: 18334032 Free PMC article. - Low numbers and no loss of melanized nigral neurons with increasing age in normal human brains from India.
Muthane U, Yasha TC, Shankar SK. Muthane U, et al. Ann Neurol. 1998 Mar;43(3):283-7. doi: 10.1002/ana.410430304. Ann Neurol. 1998. PMID: 9506543 - Cell death mechanisms in Parkinson's disease.
Jellinger KA. Jellinger KA. J Neural Transm (Vienna). 2000;107(1):1-29. doi: 10.1007/s007020050001. J Neural Transm (Vienna). 2000. PMID: 10809400 - Neuroinflammation in Alzheimer's disease and Parkinson's disease: are microglia pathogenic in either disorder?
Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A. Rogers J, et al. Int Rev Neurobiol. 2007;82:235-46. doi: 10.1016/S0074-7742(07)82012-5. Int Rev Neurobiol. 2007. PMID: 17678964 Review. - The role of iron in senescence of dopaminergic neurons in Parkinson's disease.
Youdim MB, Riederer P. Youdim MB, et al. J Neural Transm Suppl. 1993;40:57-67. J Neural Transm Suppl. 1993. PMID: 8294901 Review.
Cited by
- The acute effects of antimicrobials and lipopolysaccharide on the cellular mechanisms associated with neurodegeneration in pubertal male and female CD1 mice.
Esposito P, Gandelman M, Rodriguez C, Liang J, Ismail N. Esposito P, et al. Brain Behav Immun Health. 2022 Oct 28;26:100543. doi: 10.1016/j.bbih.2022.100543. eCollection 2022 Dec. Brain Behav Immun Health. 2022. PMID: 36345322 Free PMC article. - Glial phagocytic clearance in Parkinson's disease.
Tremblay ME, Cookson MR, Civiero L. Tremblay ME, et al. Mol Neurodegener. 2019 Apr 5;14(1):16. doi: 10.1186/s13024-019-0314-8. Mol Neurodegener. 2019. PMID: 30953527 Free PMC article. Review. - Therapeutic Inhibition of the Complement System in Diseases of the Central Nervous System.
Carpanini SM, Torvell M, Morgan BP. Carpanini SM, et al. Front Immunol. 2019 Mar 4;10:362. doi: 10.3389/fimmu.2019.00362. eCollection 2019. Front Immunol. 2019. PMID: 30886620 Free PMC article. Review. - Microglia and Parkinson's disease: footprints to pathology.
Lazdon E, Stolero N, Frenkel D. Lazdon E, et al. J Neural Transm (Vienna). 2020 Feb;127(2):149-158. doi: 10.1007/s00702-020-02154-6. Epub 2020 Feb 3. J Neural Transm (Vienna). 2020. PMID: 32016606 Review. - Transcriptomic profiling of early synucleinopathy in rats induced with preformed fibrils.
Patterson JR, Kochmanski J, Stoll AC, Kubik M, Kemp CJ, Duffy MF, Thompson K, Howe JW, Cole-Strauss A, Kuhn NC, Miller KM, Nelson S, Onyekpe CU, Beck JS, Counts SE, Bernstein AI, Steece-Collier K, Luk KC, Sortwell CE. Patterson JR, et al. NPJ Parkinsons Dis. 2024 Jan 3;10(1):7. doi: 10.1038/s41531-023-00620-y. NPJ Parkinsons Dis. 2024. PMID: 38172128 Free PMC article.
References
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann NY Acad Sci. 2003;991:120–31. - PubMed
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–91. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous