PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2 - PubMed (original) (raw)
. 2006 Nov;116(11):2869-79.
doi: 10.1172/JCI28775. Epub 2006 Oct 19.
Affiliations
- PMID: 17053833
- PMCID: PMC1616195
- DOI: 10.1172/JCI28775
PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2
Dailing Mao et al. J Clin Invest. 2006 Nov.
Abstract
Excessive bone loss in arthritic diseases is mostly due to abnormal activation of the immune system leading to stimulation of osteoclasts. While phospholipase Cgamma (PLCgamma) isoforms are known modulators of T and B lymphocyte-mediated immune responses, we found that blockade of PLCgamma enzymatic activity also blocks early osteoclast development and function. Importantly, targeted deletion of Plcg2 in mice led to an osteopetrotic phenotype. PLCgamma2, independent of PLCgamma1, was required for receptor activator of NF-kappaB ligand-induced (RANKL-induced) osteoclastogenesis by differentially regulating nuclear factor of activated T cells c1 (NFATc1), activator protein-1 (AP1), and NF-kappaB. Specifically, we show that NFATc1 upregulation is dependent on RANKL-mediated phosphorylation of PLCgamma2 downstream of Dap12/Fc receptor gamma (Dap12/FcRgamma) receptors and is blocked by the PLCgamma inhibitor U73122. In contrast, activation of JNK and NF-kappaB was not affected by U73122 or Dap12/FcRgamma deletion. Interestingly, we found that in osteoclasts, PLCgamma2 formed a complex with the regulatory adapter molecule GAB2, was required for GAB2 phosphorylation, and modulated GAB2 recruitment to RANK. Thus, PLCgamma2 mediates RANKL-induced osteoclastogenesis and is a potential candidate for antiresorptive therapy.
Figures
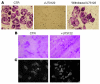
Figure 1. PLCγ inhibition blocks osteoclastogenesis and actin ring formation.
(A) WT OCs were generated with RANKL (100 ng/ml) and M-CSF (10 ng/ml) in the presence of the PLC inhibitor U73122 (5 μM) for 4 days on plastic. In some wells, the media with the inhibitor was replaced with fresh osteoclastogenic media, and cells were allowed to differentiate for 4 more days (Withdrawal U73122). (B) WT OCs were grown on dentin with or without U73122 for 10 days. Cells were then removed and pits stained with hematoxylin red (Magnification, ×200). CTR, control. (C) WT OCs were generated on dentin in the absence of the inhibitor, then treated with vehicle or U73122 for 1 hour. Cells were fixed and actin stained using FITC-phalloidin (Magnification, ×200).
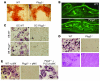
Figure 2. Osteopetrosis in mice lacking PLCγ2.
(A) TRAP staining of decalcified histological sections of WT and Plcg2–/– proximal femurs. Magnification, ×40. (B–E) Quantitative analysis of bone parameters from histological sections of WT and Plcg2–/– bone femurs (n = 8) showing: percentage of bone volume versus total bone volume (BV/TV) (B); number of OBs (nOB) per bone perimeter (C); number of OCs (nOC) per bone perimeter (D); fraction of trabecular surface covered by OCs (E). (F) 3D reconstitution of μCT scans of WT and Plcg2–/– femurs. (G–I) 3D trabecular quantitative parameters of bone structure (n = 6). Graphs show mean ± SEM, with significant differences compared with WT indicated.
Figure 3. PLCγ2 is required for osteoclastogenesis.
(A) Bone nodule formation in WT and Plcg2–/– OBs cultured with ascorbic acid and β-glycerolphosphate. (B) Double labeling of calvarial bones showing the degree of bone formation (BFR) of WT and Plcg2–/– mice injected on day 0 and day 7 with calcein (WT BFR, 0.8938 ± 0.1042 μm3/μm2/d; WT mineral apposition rate [MAR], 0.5927 ± 0.0219 μm/d; Plcg2–/– BFR, 0.8450 ± 0.1629 μm3/μm2/d; Plcg2–/– MAR, 0.4015 ± 0.0876 μm/d). (C) WT and Plcg2–/– BMMs cultured with WT and Plcg2–/– OBs in the presence of 10–8 M 1,25 Vit D3. After 14 days, cells were fixed and TRAP stained to detect the presence of multinucleated OCs. (D) TRAP-stained OCs generated with RANKL (100 ng/ml) and M-CSF (10 ng/ml) for 5 days (top panel) and bone resorptive pits generated from OCs plated on dentin for 7 days. Pits were stained with hematoxylin red. (E) WT and Plcg2–/– BMMs retrovirally transduced with vector alone (pMX) or with Flag-tagged PLCγ2 were allowed to differentiate in osteoclastogenic media for 5 days, then stained for TRAP. Magnification, ×200 (A, C–E); ×100 (B).
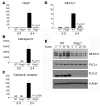
Figure 4. PLCγ2 regulates NFATc1 levels and the expression of early osteoclastogenic genes.
(A–D) Real-time PCR analysis of osteoclastogenic markers in WT and Plcg2–/– cells in culture with M-CSF alone (d0) or with RANKL (100 ng/ml) plus M-CSF (10 ng/ml) for 4 days (d4). Data are normalized relative to β-actin. ND, not detected. Significant differences compared with WT are shown. (E) Expression levels of NFATc1 protein in total cell lysates from day 4 WT and Plcg2–/– OCs, starved for 6 hours and stimulated with RANKL for 0, 5, 20, and 60 minutes. PLCγ1 and PLCγ2 levels are shown. β-Actin served as loading control.
Figure 5. PLCγ2 modulates RANKL-mediated signaling.
(A) Activation of ERK and JNK in WT and Plcg2–/– BMMs in response to M-CSF (100 ng/ml). PLCγ1 and PLCγ2 levels are shown. (B) Western blot analysis of phospho-ERK, phospho-JNK, phospho–c-Jun, and phospho-IκBα in total cell lysates from WT and Plcg2–/– BMMs stimulated with RANKL (100 ng/ml) for the indicated times. β-Actin blots served as loading control for A and B. (C) Nuclear levels of phospho–c-Jun and p65 in nuclear extracts from WT and Plcg2–/– BMMs stimulated with RANKL. (D) The same samples used in C were subjected to AP1 and NF-κB nonradioactive EMSA analysis. To control for binding specificity, a concentration of unlabeled oligonucleotides (U.O.) 200-fold greater than that recommended by the manufacturer was added to nuclear extracts from WT cells stimulated with RANKL for 60 minutes. SP1 served as loading control for C and D.
Figure 6. PLCγ2 is activated by RANKL via Dap12/FcRγ in an SFK-dependent manner.
(A) WT OC precursors (preOCs; BMMs grown in RANKL-containing media for 2 days) cultured with the SFK inhibitor PP2 (5 μM) or vehicle (DMSO) were stimulated with RANKL and subjected to Western blot analysis to detect phosphorylated levels of PLCγ2, Src, and NFATc1. β-Actin served as control. (B) PLCγ1 and PLCγ2 phosphorylation in response to RANKL were measured by Western blot analysis in WT and Plcg2–/– preOCs. PLCγ1 and PLCγ2 levels are shown. (C) PLCγ1 and PLCγ2 phosphorylation in response to 5 minutes of treatment with either M-CSF or RANKL in WT and Plcg2–/– preOCs. β-Actin served as control. (D) Expression levels of endogenous Dap12 and Flag-tagged Dap12 retrovirally transduced in Dap12–/–FcR_γ_–/– BMMs are shown. ΔKO, Dap12–/–FcR_γ_–/–. (E) PLCγ2 phosphorylation was measured by Western blot analysis in WT, Dap12–/–FcR_γ_–/–, or Dap12–/–FcR_γ_–/– preOCs reconstituted with WT Dap12 stimulated with RANKL for the indicated times. Phospho-JNK is also shown. β-Actin served as loading control. (F) Nuclear localization of NFATc1 in WT and Dap12–/–FcR_γ_–/– OCs retrovirally transduced with pMX or Flag-tagged Dap12 is shown in red (left panels). Actin staining is shown in green, and nuclei, stained with DAPI, are shown in blue (right panels) (objective, ×20). Enlarged images (2.5-fold) show nuclear localization of NFATc1 (red) and nuclei stained with DAPI (blue) of representative cells located in the center of the photographed field.
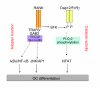
Figure 9. PLCγ2 in RANKL signaling.
PLCγ2 is phosphorylated by RANKL in an SFK-dependent manner downstream of Dap12/FcRγ. Activation of PLCγ2 and PLCγ2 catalytic activity are required for NFATc1 upregulation (right arm). PLCγ2 can also bind GAB2 and modulate its activation and recruitment to the RANK-signaling complex (left arm). This interaction might be required for activation of the IκBα/NF-κB and JNK/AP1 pathways independent of Dap12/FcRγ.
Figure 8. TNF-α cannot correct the Plcg2–/– OC defect.
(A) WT and Plcg2–/– BMMs were stimulated with TNF-α (10 ng/ml) with time. Phosphorylated JNK, IκBα, and PLCγ2 were examined. β-Actin served as control. (B) WT and Plcg2–/– BMMs were cultured for 3 days with RANKL (100 ng/ml) and M-CSF (10 ng/ml). On day 3 TNF-α was also added to the culture media. Cells were fixed and TRAP stained at day 7. Magnification, ×200. (C) WT and Plcg2–/– preOCs were stimulated with TNF-α or RANKL for the indicated times, and expression levels of NFATc1, PLCγ2, and β-actin were determined.
Figure 7. PLCγ2 forms a complex with GAB2 and modulates its activation.
(A) WT preOCs cultured with or without the PLC inhibitor U73122 (5 μM) for 3 days were stimulated with RANKL and subjected to Western blot analysis for phospho-JNK, phospho-IκBα, and NFATc1. β-Actin served as control. (B) WT and Plcg2–/– BMMs retrovirally transduced with empty vector (pMX), WT PLCγ2, or catalytically inactive PLCγ2 (PLCγ2 H/F) were cultured with RANKL (100 ng/ml) and M-CSF (10 ng/ml) for 7 days, and multinucleated OCs were detected by TRAP staining. Objective, ×10. (C) The same cells as shown in B were subjected to RANKL stimulation and Western blot analysis to detect NFATc1 expression. (D) Plcg2–/– BMMs retrovirally transduced with pMX, WT PLCγ2, or PLCγ2 H/F were treated with RANKL, and phosphorylation of IκBα and JNK was determined by Western blot analysis. PLCγ2 expression levels are shown. β-Actin served as loading control in C and D. (E) PLCγ2 and GAB2 were reciprocally immunoprecipitated in WT and Plcg2–/– BMMs treated with RANKL and subjected to Western blot analysis using anti-PLCγ2 and anti-GAB2 Abs, respectively. TCL, total cell lysate. (F) GAB2 was immunoprecipitated in WT and Plcg2–/– BMMs and subjected to Western blot analysis using anti-phosphotyrosine Ab (clone 4G10), anti-RANK, and anti-PLCγ2.
Similar articles
- Targeted inhibition of phospholipase C γ2 adaptor function blocks osteoclastogenesis and protects from pathological osteolysis.
Decker C, Hesker P, Zhang K, Faccio R. Decker C, et al. J Biol Chem. 2013 Nov 22;288(47):33634-33641. doi: 10.1074/jbc.M113.477281. Epub 2013 Sep 30. J Biol Chem. 2013. PMID: 24081142 Free PMC article. - Fyn positively regulates the activation of DAP12 and FcRγ-mediated costimulatory signals by RANKL during osteoclastogenesis.
Kim HS, Kim DK, Kim AR, Mun SH, Lee SK, Kim JH, Kim YM, Choi WS. Kim HS, et al. Cell Signal. 2012 Jun;24(6):1306-14. doi: 10.1016/j.cellsig.2012.02.014. Epub 2012 Feb 25. Cell Signal. 2012. PMID: 22387224 - Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways.
Wu X, Li Z, Yang Z, Zheng C, Jing J, Chen Y, Ye X, Lian X, Qiu W, Yang F, Tang J, Xiao J, Liu M, Luo J. Wu X, et al. J Bone Miner Res. 2012 Jun;27(6):1298-1308. doi: 10.1002/jbmr.1576. J Bone Miner Res. 2012. PMID: 22337253 - Mechanistic insight into osteoclast differentiation in osteoimmunology.
Takayanagi H. Takayanagi H. J Mol Med (Berl). 2005 Mar;83(3):170-9. doi: 10.1007/s00109-004-0612-6. Epub 2005 Jan 26. J Mol Med (Berl). 2005. PMID: 15776286 Review. - A Comprehensive Review of Immunoreceptor Regulation of Osteoclasts.
Humphrey MB, Nakamura MC. Humphrey MB, et al. Clin Rev Allergy Immunol. 2016 Aug;51(1):48-58. doi: 10.1007/s12016-015-8521-8. Clin Rev Allergy Immunol. 2016. PMID: 26573914 Free PMC article. Review.
Cited by
- A Porphyromonas gingivalis hypothetical protein controlled by the type I-C CRISPR-Cas system is a novel adhesin important in virulence.
Irfan M, Solbiati J, Duran-Pinedo A, Rocha FG, Gibson FC 3rd, Frias-Lopez J. Irfan M, et al. mSystems. 2024 Mar 19;9(3):e0123123. doi: 10.1128/msystems.01231-23. Epub 2024 Feb 7. mSystems. 2024. PMID: 38323815 Free PMC article. - Transcription Factor CTIP2 Maintains Hair Follicle Stem Cell Pool and Contributes to Altered Expression of LHX2 and NFATC1.
Bhattacharya S, Wheeler H, Leid M, Ganguli-Indra G, Indra AK. Bhattacharya S, et al. J Invest Dermatol. 2015 Nov;135(11):2593-2602. doi: 10.1038/jid.2015.281. Epub 2015 Jul 15. J Invest Dermatol. 2015. PMID: 26176759 Free PMC article. - Interaction of Tumor Necrosis Factor Receptor-associated Factor 6 (TRAF6) and Vav3 in the Receptor Activator of Nuclear Factor κB (RANK) Signaling Complex Enhances Osteoclastogenesis.
Yu J, Yun H, Shin B, Kim Y, Park ES, Choi S, Yu J, Amarasekara DS, Kim S, Inoue J, Walsh MC, Choi Y, Takami M, Rho J. Yu J, et al. J Biol Chem. 2016 Sep 23;291(39):20643-60. doi: 10.1074/jbc.M116.728303. Epub 2016 Aug 9. J Biol Chem. 2016. PMID: 27507811 Free PMC article. - Nardosinone Suppresses RANKL-Induced Osteoclastogenesis and Attenuates Lipopolysaccharide-Induced Alveolar Bone Resorption.
Niu C, Xiao F, Yuan K, Hu X, Lin W, Ma R, Zhang X, Huang Z. Niu C, et al. Front Pharmacol. 2017 Sep 12;8:626. doi: 10.3389/fphar.2017.00626. eCollection 2017. Front Pharmacol. 2017. PMID: 28955231 Free PMC article.
References
- Teitelbaum S.L., Ross F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. - PubMed
- Zhang Y.-H., Heulsmann A., Tondravi M.M., Mukherjee A., Abu-Amer Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. . J. Biol. Chem. 2001;276:563–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR52921/AR/NIAMS NIH HHS/United States
- K08 AR047846/AR/NIAMS NIH HHS/United States
- P30 AR048335/AR/NIAMS NIH HHS/United States
- AR48335/AR/NIAMS NIH HHS/United States
- AR47846/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous